Neurotransmitter System Refresher
- BioSource Faculty
- Aug 5, 2023
- 19 min read
Updated: Jan 18, 2024
While the number of neurotransmitters (NTs) is unknown, more than 100 molecules have been identified (Purves, 2008). Each NT may have multiple receptors. NTs do not exert intrinsic effects since their actions depend on interactions with their receptors. The same NT can produce opposite results at different receptor subtypes (Breedlove & Watson, 2023).

Click on our narrator icon to listen to this post.
Neurotransmitter System Families
The principal neurotransmitter system families include amino acid NTs (GABA, glutamate, glycine, and histamine), amine NTs (acetylcholine, norepinephrine, epinephrine, dopamine, serotonin, and melatonin), peptide NTs, also called neuropeptides (oxytocin, substance P, and vasopressin), purines (ATP and adenosine), lipid NTs (anandamide and AG-2), and gasotransmitters (nitric oxide, carbon monoxide, and hydrogen sulfide).
This post covers Small-Molecule and Large-Molecule NTs, Ionotropic and Metabotropic Receptors, Amino Acid NTs, Amine NTs, Peptide NTs, Lipid NTs, and Gasotransmitters.
Most of the activity of local circuits of neurons involves balances between the excitatory and inhibitory effects of these chemicals, which are responsible for most of the information transmitted within the brain. In fact, there are probably no neurons in the brain that do not receive excitatory input from glutamate-secreting terminal buttons and inhibitory input from neurons that secrete either GABA or glycine. With the exception of neurons that detect painful stimuli, all sensory organs transmit information to the brain through axons whose terminals release glutamate (Carlson & Birkett, 2019, p. 77)
Dr. Robert M. Julien (2023) addressed the orthodox and simplistic one-neuron-one-NT model.
All my training in psychopharmacology has reinforced my conviction that there are fairly few unchangeable pharmacological 'facts.' Consider, for example, the “one neuron, one transmitter” hypothesis: the idea that each neuron releases only a single neurotransmitter. Indeed, most of the discussion of neurotransmitters in this chapter presumes that principle. However, research over the last decade has demonstrated that certain neurons throughout the brain are capable of releasing two or maybe even more neurotransmitters (Avalos & Sprecher, 2021). The neurotransmitters released by these neurons, referred to as dual neurotransmitter neurons, may be rapid acting (e.g., acetylcholine) or slow acting (e.g., neuropeptides). In some of these neurons, the different neurotransmitters may be packaged within the same synaptic vesicles, while in others they may be in distinct vesicles (Julien, Advokat, & Comaty, p. 56).
Small-Molecule and Large-Molecule NTs
NTs are broadly divided into two classes based on their molecular size: small-molecule NTs and larger neuropeptides. While lipid NTs are small compared to large-molecule neuropeptides, they don't quite fit into the traditional category of small-molecule NTs due to their lipid nature and unique synthesis, release, and inactivation mechanisms.
Small-molecule NTs are usually derived from dietary amino acids and consist of one or two amino acids or are derived from a simple modification of an amino acid. They include amino acids, amines, and gases.
Large-molecule NTs or neuropeptides are chains of 3 to 36 amino acids synthesized in the neuron's cell body (Purves, 2018). The main differences between small-molecule NTs and neuropeptides involve their synthesis, release, and termination of action. Small-molecule NTs are synthesized in the nerve terminal, stored in synaptic vesicles near the presynaptic membrane, and released into the synapse in response to an action potential. Once in the synapse, they can bind to postsynaptic receptors and have a rapid, direct effect on the postsynaptic cell.
On the other hand, neuropeptides are synthesized in the cell body, packaged into vesicles, and then transported down the axon to the nerve terminal. Their release requires greater stimulation than small-molecule NTs, and their effects are slower and longer-lasting. After release, neuropeptides are not taken back into the nerve terminal for reuse (as small-molecule NTs often are) but are broken down by synaptic cleft enzymes.
Moreover, small-molecule NTs typically act on ionotropic receptors (which mediate fast synaptic transmission), whereas neuropeptides typically act on metabotropic receptors (which mediate slower, longer-lasting effects).
The table below is adapted from Breedlove and Watson (2023).

Ionotropic and Metabotropic Receptors
Synaptic transmission occurs remarkably quickly, within a time frame as brief as a millisecond. This rapid sequence includes the release of NTs from presynaptic vesicles, their diffusion across the synaptic cleft, and the attachment and activation of receptors. Depending on the specific receptor, activation may commence and conclude within just a few milliseconds after the neurotransmitter binds, or it may persist for several hundred milliseconds (Julien, Advokat, & Comaty, 2023).
Ionotropic (A) and metabotropic (B) receptors recognize NT molecules. Ionotropic receptors, chemically gated or ligand-gated ion channels, directly control an ion channel. When these receptors are bound by a NT, the ion channel opens, allowing ions to flow across the membrane. This process has a rapid onset (microseconds to milliseconds) and brief (millisecond) duration.
Metabotropic receptors interact with synaptic transmitters but do not contain ion channels themselves. These receptors control an intracellular collection of molecules known as G proteins. As a result, they are often referred to as G protein-coupled receptors (GPCRs). NT binding to these receptors activates a G protein's alpha subunit, indirectly opening a nearby ion channel or initiating other biochemical reactions within the postsynaptic cell. This process has a gradual onset (milliseconds to seconds) and long (milliseconds to hours) duration. G proteins are named for their ability to bind compounds such as guanosine diphosphate (GDP), guanosine triphosphate (GTP), and other guanine nucleotides. Sometimes, the G protein itself may open ion channels; in others, it may trigger other internal chemicals to influence the ion channels.
In this signaling system, if the NT is considered the first messenger, an external ligand arriving at the cell's surface receptor, then the subsequent chemical signal activated by G proteins within the cell serves as a second messenger. Various second messengers, like cyclic adenosine monophosphate (cAMP), diacylglycerol, and arachidonic acid, can magnify and extend the effect of the primary messenger. This alters the membrane's electrical potential. A key characteristic of second-messenger systems is their capacity to amplify and sustain the synaptic signals received by a neuron. Second messengers can produce diverse and long-lasting changes in postsynaptic neurons. For example, they can initiate gene expression or protein synthesis changes resulting in synaptic changes over minutes to days. Synaptic remodeling involves changes in synaptic structure or function. Second messengers may trigger changes in synaptic strength and structure, receptor density, NT release, and synapse formation or elimination.
Approximately 80% of known NTs and hormones engage G protein-coupled receptors (GPCRs), marking them as highly significant in cellular signaling. These proteins are situated on the inner side of the neuronal membrane. When a transmitter molecule binds to the extracellular portion of a GPCR, the G protein complex's intracellular subunits separate. The alpha subunit detaches from the beta and gamma subunits, allowing both to move and regulate the activity of their specific target molecules. Depending on the cell and receptor type, this target may be a second-messenger system, an enzyme affecting an ion channel, or an ion pump (Breedlove & Watson, 2023).
Amino Acids
Most inhibitory synapses use the NTs gamma-aminobutyric acid (GABA) or glycine in the brain and spinal cord.
Gamma-Aminobutyric Acid
Gamma-aminobutyric acid (GABA) is the inhibitory NT in up to one-third of brain synapses, most frequently in local circuit interneurons. Glucose, glutamine, and pyruvate serve as GABA precursors. Following its synthesis, vesicular inhibitory amino acid transporters (VIAATs) pack GABA into synaptic vesicles. Neurons and glia have high-affinity Na+-dependent co-transporters for GABA (Purves, 2018). Two types of postsynaptic receptors, GABA-A and GABA-B, are used at GABAergic synapses. GABA-A receptors are ionotropic, whereas GABA-B receptors are metabotropic.
Glutamate
Glutamate is the primary NT for most brain activity, released by over half of all the brain's synapses. Almost all CNS excitatory neurons are glutamatergic. Since glutamate cannot cross the blood-brain barrier, neurons synthesize it from glutamine and glucose. Vesicular glutamate transporters (VGLUTs) package glutamate in vesicles. Excitatory amino acid transporters (EAATs) located in glial cells and presynaptic terminals remove glutamate from the synaptic cleft (Purves, 2018). Three ionotropic glutamate receptors are named after agonist drugs, AMPA, NMDA, and kainate. AMPA and NMDA receptors are co-expressed at most excitatory CNS synapses. NMDA receptors are primarily responsible for excitatory transmission because they produce the largest excitatory postsynaptic potentials (EPSPs) of the three. NMDA and AMPA receptors are highly concentrated in the cerebral cortex, hippocampus, basal ganglia, septum, and amygdala. The loss of prefrontal brain volume can produce a phenomenon called cortical hypofrontality, in which prefrontal cortical (PFC) metabolic activity and the performance of executive functions are compromised. Hypofrontality reduces the ventromedial PFC's ability to resist craving, anticipate consequences, and inhibit risky behavior. Hypofrontality underlies loss of control (ventromedial PFC) and relapse. Dysfunctional glutamate transmission has been implicated in schizophrenia. Descending glutamate projections have been implicated in drug craving (Julien, Advokat, & Comaty, 2023).
Presynaptic kainate receptors may modulate glutamate release, while postsynaptic receptors generate EPSPs that peak more quickly and decay more gradually than NMDA receptors. Compared with AMPA receptors, three metabotropic glutamate receptor (mGluR) classes more gradually excite or inhibit postsynaptic neurons via intracellular signaling (Purves, 2018).
Glycine
Glycine has a more limited CNS distribution than GABA, almost half of spinal cord inhibitory synapses. Mitochondrial enzymes synthesize glycine from serine. The same vesicular transporter fills vesicles with GABA or glycine. Glycine transporters remove glycine from the synaptic cleft (Purves, 2018).
Histamine
Hypothalamic neurons release histamine to nearly all parts of the brain and spinal cord. CNS histamine projections are responsible for arousal and attention, functioning like ACh and norepinephrine. Histamine also adjusts vestibular system responsiveness. The enzyme histidine decarboxylase synthesizes histamine from the amino acid histidine. The same vesicular monoamine transporters (VMATs) used by catecholamines package histamine in vesicles. No histamine transporter has been identified to date. Histamine methyltransferase and MAO jointly metabolize histamine. The four identified histamine receptors are metabotropic.
Amines
Acetylcholine
Acetylcholine (ACh) is a quaternary amine. Cholinergic neurons are clustered in the basal forebrain, including the medial septal nucleus, the nucleus of the diagonal band, and the nucleus basalis. Cholinergic neurons project to the hippocampus and amygdala and widely throughout the cerebral cortex. Cholinergic pathway graphic © Vasilisa Tsoy/Shutterstock.com.

Cholinergic pathways are involved in arousal, attention, learning, memory, motivation, muscle contraction, and REM sleep. ACh functions at the skeletal neuromuscular junctions and the neuromuscular synapse between the vagus nerve and cardiac muscle fibers. Additionally, ACh serves as a NT within the visceral motor system ganglia and at several CNS sites.
ACh is produced in nerve terminals through a reaction that involves the precursors acetyl coenzyme A (acetyl CoA), synthesized from glucose and choline. This reaction is catalyzed by the enzyme choline acetyltransferase (ChAT). A vesicular ACh transporter (VAChT) loads approximately 10,000 molecules of ACh into each cholinergic vesicle.
Unlike most other small-molecule NT, the termination of its postsynaptic actions, especially at the neuromuscular junction, is handled by the potent hydrolytic enzyme acetylcholinesterase (AChE).
ACh binds to ionotropic nicotinic and metabotropic muscarinic receptors. More than a dozen nicotinic acetylcholine receptors (nAChRs) are responsible for many of ACh's postsynaptic effects. Five muscarinic acetylcholine receptors (mAChRs) regulate the peripheral cholinergic responses in autonomic effector organs, including the heart, smooth muscle, and exocrine glands. They play a key role in the vagus nerve's heart rate slowing (Purves, 2018).
Norepinephrine
Monoamines comprise two families: catecholamines and indoleamines. Neurons synthesize monoamines from the amino acid tyrosine. Catecholamines (norepinephrine, epinephrine, and dopamine) contain a catechol nucleus, a benzene ring with two hydroxyl groups attached, and an additional amine group. Indoleamines (serotonin and melatonin) contain an indole ring structure consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Neurons use VMATS to store monoamines in vesicles (Purves, 2018).
Norepinephrine (noradrenaline) is released by two primary brainstem clusters in the locus coeruleus in the pons and the lateral tegmental area of the midbrain. Locus coeruleus projections target the cerebrum, including the cerebral cortex, limbic system, and thalamus. The cerebellum and spinal cord are also targets of noradrenergic innervation. Adrenergic pathway graphic © Vasilisa Tsoy/Shutterstock.com.

NE affects arousal, attention, orienting, sleep, wakefulness, feeding, mood, reward, pain perception, and sexual behavior. Autonomic nervous system sympathetic ganglion cells release NE as their primary peripheral NT (Breedlove & Watson, 2023).
The motor branch of the vagus signals descending neurons to release norepinephrine, which prompts spleen immune cells to release acetylcholine to macrophages to dampen inflammation (Schwartz, 2015). NE is transported into synaptic vesicles using the same VMAT responsible for dopamine's vesicular transport. Once released into the synaptic cleft, NE is removed by the norepinephrine transporter (NET). This transporter relies on sodium (Na+) and can also take up dopamine, functioning as a co-transporter for both substances (Purves, 2018).
Both NE and E act on α-and β-adrenergic metabotropic receptors.
Epinephrine
Epinephrine (adrenaline) is present in the brain in lower quantities and released by fewer neurons than other catecholamines. Adrenergic neurons are mainly found in the lateral tegmental system and the medulla and project to the hypothalamus and thalamus. Adrenergic neurons control respiration and heart function.
The enzyme synthesizing epinephrine, phenylethanolamine-N-methyltransferase, is only found in adrenergic neurons. In other respects, epinephrine metabolism is similar to norepinephrine's, including its loading into vesicles through the VMAT.
There has been no identification of a plasma membrane transporter specific to epinephrine, but the norepinephrine transporter (NET) can transport epinephrine.
Dopamine
Dopamine is distributed through the mesostriatal and mesolimbocortical pathways. The mesostriatal pathway begins in the mesencephalon (midbrain), specifically in the substantia nigra and surrounding regions. It travels upward as part of the medial forebrain bundle to connect with the striatum, including the caudate nucleus and putamen. These structures are components of the basal ganglia. The mesostriatal dopamine (DA) pathway is vital for motor control, and a significant loss of these neurons leads to the movement deficits characteristic of Parkinson's disease.
Similarly originating in the midbrain, the mesolimbocortical pathway starts in the ventral tegmental area (VTA) and extends to the limbic system, encompassing the amygdala, nucleus accumbens, hippocampus, and cortex. This system plays a significant role in reward and reinforcement processes, particularly through the dopamine D2 receptor subtype. Dopaminergic pathway graphic © Vasilisa Tsoy/Shutterstock.com.

Out of the 80 to 90 billion neurons in the human brain, around a million synthesize dopamine (Breedlove & Watson, 2023). Despite this small number, they exert a substantial influence on behavior. Dopamine is also thought to play roles in motivation, reward, and reinforcement. Many abused substances affect CNS dopaminergic pathways. Dopamine's role in some sympathetic ganglia is poorly understood.
Dopamine is synthesized in the cytoplasm of presynaptic terminals and then transported into synaptic vesicles by VMATs. Its activity in the synaptic cleft is ended by reabsorption into the nerve terminals or neighboring glial cells through a sodium (Na+)-dependent dopamine co-transporter (DAT) (Purves, 2018).
Dopamine breakdown involves monoamine oxidase (MAO) and catechol O-methyltransferase (COMT) found in neurons and glial cells. MAO is located in the mitochondria, and COMT in the cytoplasm.
After release, dopamine triggers G-protein-coupled receptors. Most dopamine receptor subtypes turn on or off adenylyl cyclase, helping regulate complex behaviors.
Serotonin
Serotonin (5-HT) is an indoleamine. Serotonergic fibers innervate large brain areas, even though their cell bodies are relatively scarce, numbering only about 200,000 in the human brain (Breedlove & Watson, 2023). These cell bodies are mainly concentrated along the midline in the raphe nuclei of the midbrain and brainstem. Despite their limited number, like dopaminergic neurons, serotonergic neurons exert a broad influence throughout the brain. Serotonergic pathway graphic © Vasilisa Tsoy/Shutterstock.com.
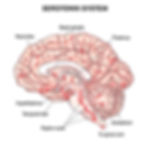
Serotonin is created from the amino acid tryptophan. It is loaded into synaptic vesicles by VMATs, which also transport other monoamines into these vesicles. Its reuptake into nerve terminals ends the effects of serotonin at the synapse through a specific serotonin transporter (SERT). This transporter is located in the presynaptic plasma membrane and is encoded by the 5HTT gene. Selective serotonin reuptake inhibitors (SSRIs) 5-HT reuptake by SERT. MAO is primarily responsible for breaking serotonin down (Purves, 2018).
To date, researchers have identified 14 5-HT receptors. Most are metabotropic and help to regulate circadian rhythms, arousal, anxiety, emotional states, pain, and motor and sexual behavior.
ATP and Other Purines
Since all vesicles contain adenosine triphosphate (ATP) and it is co-released with conventional NTs, ATP may function as a co-transmitter (Purves, 2018). ATP is co-released with GABA, glutamate, ACh, DA, and peptides (Bearm Connors, & Paradiso, 2020). ATP is a purine, containing a purine ring, and several purines are recognized NTs. ATP serves as an excitatory NT in spinal motor neurons and sensory and autonomic ganglia. Moreover, ATP's CNS postsynaptic effects have been observed within dorsal horn neurons and hippocampal neurons. Once released, ATP is broken down into adenosine by extracellular enzymes. Adenosine also functions as a non-classic NT since it is not stored in synaptic vesicles and its release does not depend on an influx of Ca2+.
ATP and adenosine receptors are widely distributed throughout the nervous system and other body tissues (Purves, 2018). Three classes of purine receptors have been identified, one is ionotropic (P2X) and two are metabotropic. P2X receptors produce excitatory postsynaptic responses, especially in sensory nerves that transmit information about mechanosensation and pain.
Peptides
Numerous peptides function as hormones and NTs. Some of these peptide transmitters, for instance, modulate emotions. Others, like substance P and opioid peptides, contribute to pain perception. Additional peptides, such as melanocyte-stimulating hormones, adreno-corticotropin, and β-endorphin, help to regulate our complex responses to stressors (Purves, 2018).
Peptide NT synthesis and packaging mechanisms significantly differ from those of small-molecule NTs. They resemble protein synthesis in non-neuronal cells (e.g., the creation of pancreatic enzymes). Peptide-secreting neurons usually generate polypeptides substantially larger than the final, "mature" peptide.
These large polypeptides, known as pre-propeptides or pre-proproteins, are processed within the neuron's cell body through a series of reactions happening in various intracellular organelles. Pre-propeptides are synthesized in the rough endoplasmic reticulum, where the signal sequence (the sequence of amino acids that indicates the peptide is destined for secretion) gets removed. The resulting polypeptide, a propeptide or proprotein, then moves through the Golgi apparatus and gets packed into vesicles in the trans-Golgi network.
The final stages of peptide neurotransmitter processing, which include proteolytic cleavage, end-modification of the peptide, glycosylation, phosphorylation, and the formation of disulfide bonds, occur post-packaging into vesicles.
Based on their amino acid sequences, neuropeptide NTs can be broadly sorted into five categories: brain/gut peptides; opioid peptides; pituitary peptides; hypothalamic-releasing hormones; and a miscellaneous group that encompasses other peptides not easily classified into the preceding groups.
Substance P
Substance P, an 11-amino-acid peptide classified as a brain/gut peptide, is abundant in the human hippocampus, neocortex, and gastrointestinal tract. It is also discharged from C fibers - the fine afferents in peripheral nerves that transmit information about pain, temperature, and postganglionic autonomic signals. As a sensory NT in the spinal cord, its release can be inhibited by opioid peptides emanating from spinal cord interneurons, leading to pain suppression (Purves, 2018).
Opioid Peptides
Researchers have identified over 20 opioid peptides within three groups: endorphins, enkephalins, and dynorphins. Each group originates from a unique inactive pre-pro-peptide (pre-proopiomelanocortin, pre-proenkephalin A, and pre-prodynorphin) linked to specific genes. Precursors are converted into active opioid peptides by enzymes in specific tissues and in the Golgi apparatus. Opioid peptides are found throughout the brain and are co-located with small-molecule NTs like GABA and 5-HT. Generally, opioids function as depressants (Purves, 2018). Opioids may play a role in acupuncture-induced pain relief, reward, and complex behaviors like sexual attraction and aggression (Breedlove & Watson, 2023). Their involvement in autism and schizophrenia is controversial. Almost all neuropeptides trigger their effects by activating G-protein-coupled receptors. Neuropeptide receptor activation helps to control the output from sympathetic ganglia and gut activity. The neuropeptide Y receptor contributes to feeding behavior, impacting satiety and obesity. Additionally, peptide receptor activation is associated with behaviors like anxiety and panic attacks (Purves, 2018).
Unconventional NTs
Several unique molecules act as signals between neurons and their targets. They qualify as NTs due to their roles in neuron-to-neuron signaling and calcium-regulated release. However, they are unorthodox compared to classical NTs because they are not stored in synaptic vesicles nor released via exocytosis. These atypical NTs are not always released from presynaptic terminals and are frequently involved in retrograde signaling (Purves, 2018). In retrograde transmission, a presynaptic neuron sends a chemical message to the postsynaptic neuron. In response, the postsynaptic neuron synthesizes and distributes an endocannabinoid (e.g., anandamide) or gas (e.g., nitrous oxide) to the presynaptic neuron and its immediate active neighbors. Neurons synthesize these NTs on demand since they cannot be contained by vesicles.
Endocannabinoids
Endocannabinoids, such as anandamide and 2-arachidonoylglycerol (2-AG), bind to the same cannabinoid receptors targeted by the psychoactive component of marijuana, ∆9-tetrahydrocannabinol (Julien, Advokat, & Comaty, 2023). Endocannabinoid production is prompted by a rise in postsynaptic Ca2+ concentration within postsynaptic neurons. This allows these signals to diffuse through the membrane to interact with cannabinoid receptors on other cells. These ligands are later reabsorbed and broken down by the enzyme fatty acid hydrolase (FAAH).
Two cannabinoid receptors have been identified, CB1 and CB2. CB1 receptors, which are G-protein-coupled, are involved in CNS endocannabinoid actions. Endocannabinoids mainly inhibit communication between presynaptic and postsynaptic cells, acting as retrograde signals to control GABA release at specific inhibitory synapses in areas like the hippocampus and cerebellum. When endocannabinoids bind to CB1 receptors on presynaptic terminals, they reduce GABA release triggered by presynaptic action potentials, diminishing inhibitory transmission (Purves, 2018).
Gasotransmitters
NO and other gasotransmitters like carbon monoxide (CO) and hydrogen sulfide (H2S) differ significantly from classical NTs.
First, gasotransmitters are produced in areas like dendrites, not just axon terminals, and they diffuse out of the neuron immediately upon production without being stored in or released from vesicles. Second, these transmitters don't interact with membrane-bound receptors on target cells; instead, they infiltrate the target cell and influence the production of second messengers. Last, gasotransmitters can function as retrograde transmitters, moving from the postsynaptic neuron to the presynaptic one, stimulating changes in synaptic efficacy involved in learning and memory (Breedlove & Watson, 2023).
Nitric oxide (NO) is a gas produced by nitric oxide synthase, an enzyme that transforms the amino acid arginine into citrulline, simultaneously generating NO. In neurons, NO synthase activity is regulated by calcium binding to the sensor protein calmodulin. Once created, NO can cross the plasma membrane, enabling it to move from its cell of origin to act within nearby cells. This gaseous signal can diffuse tens of micrometers from its production site before it breaks down. NO quickly decays by reacting with oxygen, creating inactive nitrogen oxides and resulting in short-lived signals (Purves, 2018).
NO can synchronize the activities of multiple cells within a localized area and might facilitate some types of synaptic plasticity that occur within small neuron networks. NO signaling appears to regulate synapses that also use conventional NTs, with glutamate-releasing presynaptic terminals being the most examined NO targets in the CNS.
Summary
Complex synaptic computations in neural circuits result from numerous NTs acting on more postsynaptic NT receptors. Glutamate is the main excitatory NT in the brain, while GABA and glycine are key inhibitory ones. The reactions of these small-molecule NTs are usually faster than those of neuropeptides. Therefore, small-molecule NTs often mediate rapid-response synaptic transmission. In contrast, neuropeptides, biogenic amines, and some small-molecule NTs typically moderate ongoing brain or peripheral tissue activity more slowly and persistently (Purves, 2018).
Two primary families of NT receptors, ionotropic or ligand-gated ion channels and metabotropic receptors, perform postsynaptic signaling. Ionotropic receptors, combining the neurotransmitter receptor and ion channel, enable rapid postsynaptic electrical responses. Metabotropic receptors indirectly regulate postsynaptic ion channel activity, typically via G-proteins, causing slower, longer-lasting electrical responses. These receptors play a critical role in behavior regulation, and drugs targeting them have effectively treated various behavioral disorders.
The postsynaptic response at a synapse is determined by the combination of receptor subtypes, G-protein subtypes, and ion channels in the postsynaptic cell. Given that these features can vary within and between neurons, a vast range of transmitter-mediated effects is achievable.
Quiz
Take a five-question quiz on Quiz Maker to assess your mastery.
Glossary
acetylcholine (ACh): a small-molecule NT that plays roles in attention, learning, memory, and muscle contractions.
adenosine: a purine NT that influences various physiological processes like energy transfer and signal transduction.
adenosine triphosphate (ATP): a molecule that carries energy within cells; also acts as a purine NT in the nervous system.
AG-2: a lipid NT involved in the endocannabinoid system.
amino acids: organic compounds that combine to form proteins.
amino acid neurotransmitters: NTs made from amino acids, including glutamate and glycine.
AMPA receptors: ionotropic receptors that respond to the NT glutamate, playing key roles in fast synaptic transmission.
amine neurotransmitters: NTs derived from ammonia; includes the monoamines (dopamine, norepinephrine, and serotonin) and acetylcholine.
anandamide: a lipid NT involved in the endocannabinoid system, implicated in mood, pain, appetite, and memory.
carbon monoxide: a gasotransmitter involved in modulating neuronal signaling.
catechol O-methyltransferase (COMT): an enzyme that breaks down catecholamines, such as dopamine, epinephrine, and norepinephrine.
catecholamines: a class of monoamine NTs including dopamine, norepinephrine, and epinephrine.
cortical hypofrontality: a condition characterized by decreased activity in the prefrontal cortex, associated with schizophrenia and chronic drug abuse.
dopamine (DA): A monoamine NT involved in reward, motivation, motor control, and other functions.
drug craving: an intense desire for a specific drug, often related to drug addiction.
dynorphins: opioid peptides that act as NTs in mood regulation, stress response, and pain perception.
endocannabinoids: lipid NTs that participate in the body's endocannabinoid system.
endorphins: opioid peptides that act as NTs, released during stress and physical activity, often associated with feelings of pain relief and euphoria.
enkephalins: opioid peptides that act as NTs, reducing pain and promoting relaxation.
epinephrine (adrenaline): a monoamine NT that plays a major role in stress response.
excitatory amino acid transporters (EAATs): proteins that remove glutamate from the synaptic cleft, helping to terminate its excitatory effects.
first messenger: the first step in cellular signaling, usually a hormone or NT, that binds to a receptor, leading to cellular responses.
G protein-coupled receptors (GPCRs): a class of metabotropic receptors that respond to a variety of molecules, including NTs and hormones.
gamma-aminobutyric acid (GABA): an amino acid NT that primarily functions as an inhibitory NT, reducing neuronal excitability.
gasotransmitters: a subclass of NTs that are gaseous at room temperature, such as nitric oxide, carbon monoxide, and hydrogen sulfide.
glutamate: an amino acid NT that acts as the primary excitatory NT in the nervous system.
glycine: an amino acid NT that primarily functions as an inhibitory NT in the spinal cord and brainstem.
Golgi apparatus: a cellular organelle involved in protein processing and packaging.
histamine: an amine NT involved in local immune responses as well as regulating physiological function in the gut and acting as a NT in the brain.
hydrogen sulfide: A gasotransmitter involved in a variety of processes including inflammation and neuromodulation.
indoleamines: a subclass of monoamines including serotonin and melatonin.
ionotropic receptors: fast-acting NT receptors that function as ion channels.
kainate receptors: ionotropic receptors for the NT glutamate, involved in excitatory neural transmission.
large-molecule neurotransmitters: NTs that are larger than small-molecule NTs and often composed of multiple amino acids (peptide NTs).
lipid neurotransmitters: NTs, such as endocannabinoids and anandamide, composed of lipid molecules.
melatonin: an indoleamine NT primarily released by the pineal gland, regulating sleep-wake cycles.
mesolimbocortical pathway: a major dopamine pathway in the brain involved in reward processing and motivation.
mesostriatal pathway: also known as the nigrostriatal pathway, a major dopamine pathway involved in regulating movement.
metabotropic receptors: G protein-coupled receptors that act through second messengers rather than ion channels.
monoamine oxidase (MAO): an enzyme that breaks down monoamine NTs such as dopamine, norepinephrine, and serotonin.
monoamines: a NT class that includes dopamine, norepinephrine, serotonin, and others.
neurotransmitters: chemicals that transmit signals across a synapse from one neuron to another.
nitric oxide (NO): a gasotransmitter involved in a variety of functions such as vasodilation and neurotransmission.
norepinephrine: a monoamine NT involved in alertness, arousal, and the fight-or-flight response.
norepinephrine transporter (NET): a protein responsible for reuptake of norepinephrine into presynaptic nerve terminals.
NMDA receptors: ionotropic receptors that respond to glutamate and are involved in synaptic plasticity and memory function.
opioid peptides: peptides that bind to opioid receptors in the brain; includes endorphins, enkephalins, and dynorphins.
oxytocin: a peptide NT involved in social bonding, sexual reproduction, and during and after childbirth.
P2X receptor: a type of purinergic receptor for ATP, involved in various physiological functions.
Parkinson’s disease: a long-term degenerative nervous system disorder that mainly affects the motor system, often linked with dopamine deficits.
peptides: organic compounds composed of amino acids.
peptide neurotransmitters: NTs composed of amino acids, often with longer chains than those of small-molecule NTs.
pre-propeptide: the precursor to a propeptide, a longer protein that will be cleaved to form the final peptide product.
propeptide: a peptide that is cleaved from a proprotein and can sometimes function as a NT or hormone.
proprotein: the precursor to a protein, often cleaved to form one or more final protein products.
purine: a type of organic molecule that forms the building blocks of DNA and RNA.
purine neurotransmitters: NTs, such as adenosine and ATP, that are purines, a type of organic molecule.
retrograde transmission: communication in the nervous system where signals move in the opposite direction from the usual synaptic transmission, often associated with endocannabinoids and gasotransmitters.
second messenger: intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers.
serotonin: a monoamine NT involved in mood regulation, digestion, sleep, and many other functions.
small-molecule neurotransmitters: a NT class, typically derived from dietary amino acids, that include biogenic amines and amino acids.
sodium (Na+)-dependent dopamine co-transporter (DAT): a protein responsible for the reuptake of dopamine from the synapse back into the neuron.
specific serotonin transporter (SERT): a monoamine transporter protein responsible for the reuptake of serotonin into presynaptic cells.
Substance P: a peptide NT involved in pain perception and mood regulation.
synaptic remodeling: the process by which neurons reorganize their connections, key to learning and memory.
tryptophan: an essential amino acid that serves as a precursor to the NT serotonin.
vasopressin: a peptide NT (also known as antidiuretic hormone) involved in water regulation in the kidneys and social bonding.
vesicular glutamate transporters (VGLUTs): proteins that package glutamate into vesicles for release into the synaptic cleft.
References
Bear, M., Connors, B., & Paradiso, M. A. (2020). Neuroscience: Exploring the brain, Enhanced Edition (4th ed.). Jones & Bartlett Learning. Breedlove, S. M., & Watson, N. V. (2023). Behavioral neuroscience (10th ed.). Sinauer Associates, Inc. Carlson, N. R., & Birkett, M. A. (2019). Foundations of behavioral neuroscience (10th ed.). Pearson Education. Julien, R., Advokat, C. D., & Comaty, J. E. (2023). Julien's primer of drug action (15th ed.). Macmillan Higher Education. Purves, D. (2018). Neuroscience (6th ed.). Oxford University Press Academic. Schwartz, S. (2015). Viva vagus: Wandering nerve could lead to range of therapies. Science News, 188(11), 18.
Learn More




