Remodeling Brain Networks with Neurofeedback
- BioSource Faculty
- Aug 4, 2024
- 24 min read
Updated: Jun 23, 2025

This post will describe the brain's job and how it performs it through networks. We will review examples of brain network involvement in psychological functions and dysfunctions. We will explain how neurofeedback and biofeedback can remodel dysfunctional brain networks to ease psychological and behavioral challenges. Understanding brain network structure and function should inform the selection of neurofeedback protocols.
What Does the Brain Do?
The brain is the organ of exploration, adaptation, homeostasis, and consciousness. That is, the brain supports stable biological health that is adaptive to the changing demands of the environment as the person explores the world in the service of self- and species preservation (Buzsáki, 2019; Llinás, 2001). Barrett (2020) argued that the brain does more than react to stimuli. Based on earlier learning, its search and exploration functions predict events and anticipate consequences. Why does it expend so much energy to do so? To maintain physiological balance and health and ensure its survival. This function is termed allostasis (Ganzel et al., 2010; Sterling, 2014).
In our summary of Lisa Feldman Barrett's chapter, The Brain Is Not for Thinking, we wrote:
Allostasis refers to the brain's ability to predict and prepare for the body's needs before they arise. Unlike homeostasis, which maintains balance in response to changes, allostasis anticipates needs and adjusts the body's internal state accordingly. This predictive function is central to the brain's role in managing the body's energy budget. For instance, if a creature senses a potential predator, the brain prepares the body for a quick escape even before the threat becomes imminent. This ability to predict and respond efficiently is crucial for survival. Barrett emphasizes that allostasis is a fundamental brain function, illustrating its primary role as an energy management system rather than a thinking organ. The brain constantly balances the body's resource intake and expenditure, ensuring it functions optimally in various environmental conditions.
The brain then generates behaviors to achieve allostasis that occur in particular environmental conditions and are made more or less likely in the future by the consequences that follow those behaviors.
How Does the Brain Do It?
Holistic Views of the Brain
Bem et al. (2021) summarized that Alcmaeon of Croton, around 500 BC, identified the brain as the origin of thinking. Karl Lashley (1930) proposed a holistic approach to the brain in which higher mental functions relied on mass action, deemphasizing localization of function.
Phrenology
Models of brain function have evolved as science has progressed; for example, it has emphasized the localization of function, especially with the development of phrenology by Franz Gall in the late 18th century (Finger, 2009). Phrenology asserted that different parts of the brain had unique functions, contrasted with earlier thought that the brain operated as an undifferentiated whole. Connections between areas were mostly ignored. Phrenology head statue graphic © life_in_a_pixel/Shutterstock.com.

Localization of Function in 19th-Century European Neurology
Paul Broca and Carl Wernicke, among others in France and Germany, began using the lesion method to study individuals who had sustained brain injury and lost an ability like speech. This provided correlational evidence that the damaged area was needed for intact function. This furthered the theories of brain localization for specific functions. The language area graphic was uploaded to ResearchGate by Zouhair Khatouri (2021).

However, even in the 19th Century, regions of interest were seen as connected in networks (Bullmore & Vértes, 2013). For example, the French neurologist Déjerine described the condition of alexia without agraphia (i.e., the inability to read but with preserved ability to write) as resulting from a lesion of the splenium of the corpus callosum that disconnected the left and right posterior regions of the brain (Cheema & Chen, 2020).

Brodmann Areas
In the early 20th Century, Korbinian Brodmann (2006) conducted exhaustive studies of cortical neuron types and their architectural arrangements. This cytoarchitectonic exploration identified over 50 different areas based on their anatomical and microstructural elements. Various mental functions have been associated with these areas (Strozer, 2009), and the relationship of Brodmann Areas to EEG electrode sites in the 10-20 system has been presented (Vieito, da Rocha, & Rocha, 2015).

Brodmann area graphic © sciencepics/Shutterstock.com.

Connection
However, Brodmann areas and regions of interest (ROIs, anatomically identified structures that overlap with BAs; e.g., BA46 is roughly the dorsolateral prefrontal cortex) are connected to each other and to noncortical areas (e.g., Baker et al., 2018; Ryyppö et al., 2018).
More generally, the structures and regions of interest that make up the central nervous system are connected to each other, and function in the service of sensation and movement, cognition, emotion, operation of the body's various systems (e.g., skeletal, muscular, nervous, endocrine, cardiovascular, lymphatic, respiratory, digestive, urinary, and the reproductive), as well as allostasis, as noted by Barrett (2020). The pairwise functional connection image below is from Hearne et al. (2016).

Fiber tract graphic © Connect Images/Shutterstock.com.

Caption: The splenium and genu of the corpus callosum, the fornix, and the cingulum bundle.
Networks
As described by Lisa Feldman Barrett in her 2020 book Seven and a Half Lessons About the Brain, brain networks are organized into connected hubs and clusters that communicate information efficiently. The same hub or node may be involved in more than one network.
CNS structures are connected into network activity (e.g., the ROIs of Boca's Area/pars triangularis/BA 45 via the arcuate fasciculus to Wernicke's Area/superior temporal gyrus/BA22 for language). When activated, the network serves particular sensory, motor, cognitive, language, emotional, and somatic functions. Different groups of brain networks have been identified through neuroanatomic, MRI and fMRI mapping (e.g., Hagmann et al., 2008; Laird et al., 2011).
Examples are executive control, salience, default mode, and language networks. As described by Pessoa (2014), different brain networks are composed of overlapping structures and are dynamically recruited based on context or the situational demands a person faces. For example, the salience network recruits and activates the executive control network (Pessoa, 2014). Salience graphic by van Ettinger-Veenstra et al. (2019).

Caption: Node connections of the default mode network and the salience network. Cartoon of node connections used in this study as seen on a see-through brain. Green shows the connections between the nodes of the DMN, and orange shows the connection between the nodes of the SN.
CNS structures have been organized into several different sets of networks; there is not one definitive list of brain networks, although some networks (e.g., default mode network) are identified as canonical because they appear repeatedly in research from multiple laboratories (e.g., Yeo et al., 2011; visual, somatomotor, dorsal attention, ventral attention, limbic, frontoparietal, default).
Whereas structural connectivity in the brain refers to how ROIs are physically connected by tracts and fasciculi (e.g., Broca's area is physically connected to Wernicke's area by the arcuate fasciculus), functional connectivity refers to how distinct regions of the brain are co-activated to serve a given function (e.g., executive functions), whether or not there is an apparent direct neural route between regions. Functional connectivity is studied mainly by presenting tasks while measuring brain activity using fMRI or PET imaging. Dynamic functional connectivity is the label for how the configurations of connected brain regions flexibly change based on the person's situational needs (Hutchinson et al., 2010; Li et al., 2023). One network may also mediate the switching from a second to a third network (e.g., the salience network switches the brain from the attention network to the executive network; Menon & Uddin, 2010).
Brain networks can also be described by their effective connectivity, directionality, or how one node influences another.
Networks are also organized in a so-called small-world manner. Imagine a diagram of hubs that connect to many nodes, with nodes connected to a few other nodes so that nodes cluster together so that distances between them are short. This small world arrangement lets the network work with its interacting elements with high efficiency and adaptability to changing conditions inside and outside the body. As described in our July 15, 2024 post, Barrett (2020) reasoned that the brain's ability to integrate information from various sources is crucial for survival, enabling us to predict and respond to our environment effectively.
Brain networks connect structures of the cortex, cortical and subcortical structures, CNS structures with the cardiac nervous system, and CNS structures with the enteric nervous system by using neural and hormonal signals.
Types of Networks
Medaglia (2019) categorized nervous system networks as either structural or functional. Structural (anatomical) networks are based on methods using diffusion imaging and tractography algorithms to represent network connections graphically and use graph theoretical analyses. Functional networks are based on methods that use fMRI, EEG, MEG, ECoG (electrocorticography), and multi-electrode recordings (Yao et al., 2015). However, the terminology for networks is inconsistent at this time.
Structural Networks
Structural networks shape the connectivity of functional networks. Catani and Thibault de Schotten (2012) present an atlas of anatomical connections in the human brain. For example, they present the direct (arcuate fasciculus) and indirect (anterior and posterior segments) that connect Broca's and Wernicke's areas in the left perisylvian pathways that serve language functions. Disorders associated with the left perisylvian pathway relate to dysphasia, with the right perisylvian pathway to left hemifield neglect. The brain network connectome graphic below is from Bañuelos and Verstynen (2019).

Functional Networks
Functional networks have been organized into groups with larger or smaller numbers of networks. For example, Schimmelpfennig and colleagues (2023) identified three canonical brain networks (default mode, salience, frontoparietal) based on findings that play a role in many cognitive processes and psychiatric conditions. The figure below is from Schimmelpfennig and colleagues (2023).

Uddin, Yeo, and Spreng (2019) used a taxonomy of six functional brain networks.

Caption: In the authors' proposed taxonomy, networks are referred to by anatomical names that best describe six ubiquitous large-scale functional systems. The names in blue refer to the broad cognitive domains with which a given anatomical system is most commonly associated. Only 1-2 core nodes of each network are depicted here, though it is understood that multiple additional cortical, subcortical, and cerebellar nodes may be affiliated with a given network.
Hagmann et al. (2008) identified hubs comprising the CNS's core, including six networks.

Caption: The modularity was derived from the average regional connection matrix. Modules are listed in Table S1. (A) The plot shows a dorsal view, with nodes representing anatomical subregions. Six modules are shown as gray circles centered on their center of mass and sized according to their number of members. (B) Connector hubs obtained from analyses of high-resolution connection matrices. ROIs are displayed according to how consistently a given ROI was identified as a connector hub across participants.
Boerger et al. (2023) proposed anatomical topographies of large-scale networks.

Furthermore, networks for particular processes have been identified. For example, Procelli et al. (2018) describe five networks important for social behavior, classifying these as amygdala or non-amygdala networks.

Resting State Networks
Seitzman and colleagues (2019) provided an overview of so-called resting state networks (AKA intrinsic connectivity networks, ICNs), collections of brain regions that show synchronous (correlated) activity.

Laird et al. (2011) identified 20 intrinsic connectivity networks.

Graphic by Gonzalez-Madruga et al. (2022). The affective network is designated by AN.

Caption: Illustration of six resting-state networks. The default mode (DMN), affective (AN), frontoparietal control (FPCN), dorsal attention (DAN), salience (SN), and the somatomotor/sensory networks (SMN). Arrows reflect findings of within and between network hypo-and hyperconnectivity in individuals with ADHD as identified by Gao et al. (2019).
Brain networks can be examined based on connectivity within the cortex, between the cortex and subcortical nuclei, between the brain and the intrinsic cardiac nervous system, and between the brain and the enteric nervous system. These networks are important for cognition as well as physical and mental health.
Cortico-Cortical Examples
Three canonical cortical networks are the salience, central executive, and default mode networks (Sridhara, Levitin, & Menon, (2008). Seven-network graphic by Feirrera et al. (2022). The Executive Network is shown in blue.

Caption: Brain networks: One atlas with seven networks. Seven brain networks derived from resting‐state fMRI data were adapted from Schaefer et al. (2018).
Salience Network
The salience network's main cortical hubs are the anterior insula and dorsal anterior cingulate. Its main function is to detect salient stimuli and switch from one network to another, especially from the default mode network to the central executive network.
Central Executive Network
According to Heinonen et al. (2016), the most important parts of the executive network are the dorsolateral prefrontal cortex (DLPFC) and the anterior cingulate cortex (ACC). The executive network involves working memory functions, decision-making, and problem-solving in the service of goal-directed behavior (Menon, 2011).
Default Mode Network
Raichle (2015) identified the DMN as being comprised of "discrete, bilateral and symmetrical cortical areas, in the medial and lateral parietal, medial prefrontal, and medial and lateral temporal cortices" (p. 433). Its activation involves self-referential thought, that is, thinking about matters related to oneself. Shim G, Oh JS, Jung WH, et al., CC BY 3.0, via Wikimedia Commons.

Caption: Default mode and task-related maps for healthy subjects. On a green background, the default mode network is highlighted in warm colors (red and yellow), and the task-related network is highlighted in cold colors (blue and light blue) depending on the p-value of a one-sample t-test.
The following figure from Sridhara et al. (2008) shows the main cortical regions of interest for the salience, central executive, and default mode networks.

Cortical-Subcortical Examples
Tekin and Cummings (2002) outlined five frontal-subcortical circuits of importance for neuropsychiatry. These circuits are connected in parallel to link specific frontal cortex regions to the striatum, basal ganglia, thalamus, and back to the originating area of the frontal cortex.

Lesions of the dorsolateral circuit are associated with executive dysfunction. In contrast, lesions of the orbitofrontal circuit are related to disinhibited personality change, and those of the anterior cingulate are correlated with apathy. The orbitofrontal cortex is part of the mentalizing network. Graphic courtesy of Han and colleagues (2021).

Caption: The orbitofrontal cortex (OFC) is designated by a blue circle.
CNS-Cardiac Examples
Corcoran et al. (2023) present a diagram of connections between the central nervous system and the intrinsic cardiac nervous system. Cortical regions of interest include the anterior cingulate cortex, ventromedial prefrontal cortex, and the insula.

Intrinsic cardiac nervous system graphic courtesy of the Institute of HeartMath.

CNS-Enteric Examples
The enteric nervous system is a branch of the autonomic nervous system that innervates the digestive system. This system is connected to the central nervous system and microbiome tissue system. The interactions between these systems help explain how behavioral treatments such as cognitive behavior therapy, for example, can positively affect conditions such as irritable bowel syndrome (Mayer, Ryu, & Bhatt, 2023).
Jeanne and colleagues (2023) have recently conducted a study involving heart rate variability (HRV) biofeedback to explore its ability to modulate gut-brain coupling. Results showed decreased tonic and phasic sympathetic activity, increased EEG alpha power, and decreased delta power.
The Initiative for Neural Science, Disease & Engineering at Tufts University posts the diagram below on their website: https://sites.tufts.edu/INSciDE/gut-brain-axis.

Dynamics: How Networks Network With Each Other
Braun et al. (2021) concluded that cognitive tasks require dynamic brain state transitions between functional networks. They wrote, "it remains unclear how the brain controls, steers, and guides transitions between these different states and which brain components underlie the coordinated adaptation of brain-wide activity patterns" (p. 2). Network interaction graphic from Bañuelos and Versteynen (2019).

Network Problems in Neurology and Psychiatry: Diagnoses and Their Network Correlates
Four examples are given below of network function in mental health conditions: depression, generalized anxiety disorder, PTSD, and ADHD.
Depression
Li et al. (2018) present a network model for depression comprised of four overlapping networks, each of which is linked to symptoms of depression.

They summarized their review article by stating:
Elevated connectivity of a ventral limbic affective network appears to be associated with excessive negative mood (dysphoria) in the patients; decreased connectivity of a frontal‐striatal reward network has been suggested to account for loss of interest, motivation, and pleasure (anhedonia); enhanced default mode network connectivity seems to be associated with depressive rumination; and diminished connectivity of a dorsal cognitive control network is thought to underlie cognitive deficits especially ineffective top‐down control of negative thoughts and emotions in depressed patients. Moreover, the restoration of connectivity of these networks—and corresponding symptom improvement—following antidepressant treatment (including medication, psychotherapy, and brain stimulation techniques) serves as evidence for the crucial role of these networks in the pathophysiology of depression (p. 1004).
Generalized Anxiety Disorder (GAD)
Li et al. (2020) compared patients with generalized anxiety disorder (GAD) with healthy control subjects (HC). They found evidence of diminished top-down control in the brain function of GAD patients related to hyperconnection of the insula, suggesting difficulty discriminating between safety and threat, and intolerance of uncertainty.

Post-Traumatic Stress Disorder (PTSD)
Ross and Cisler (2020) reviewed fMRI methods used to study PTSD and their various findings. They write that this group of patients shows reduced functional connectivity, especially in the default mode network (DMN). The figure below depicts connectivity in the cortical and subcortical structures related to DMN function (Alves et al., 2019).

Attention-Deficit Hyperactivity Disorder (ADHD)
Luo et al. (2023) conducted an fMRI study of subjects with ADHD. They correlated the dynamic functional connectivity (dFC) in various brain networks with four clusters of symptoms: inattention/hyperactivity, somatization, inhibition and flexibility, and fluency and memory. Different patterns of dFC emerged based on the symptom cluster. However, the dFC in the default mode network (DMN), sensorimotor network, and fronto-parietal networks were most involved, with dFC in the DMN implicated in each symptom cluster.

How to Target Networks Using Neurofeedback and Biofeedback
Examples of how research findings of network dysrhythmias in mental health conditions can be integrated with client-derived information and neurofeedback research findings are given below for depression, GAD, PTSD, and ADHD.
With respect to network findings for conditions such as depression, GAD, PTSD, and ADHD, an implication for EEG neurofeedback is that qEEG assessment of network function may lead to the identification of dysrhythmias of cortical sites and networks found to be of concern for each of these disorders. Cortical sites and/or networks that are functioning out of the norm could be effectively addressed with 19-channel swLORETA-type methods. Graphic courtesy of NeuroNavigator’s swLORETA Effective Connectivity Normative Database.
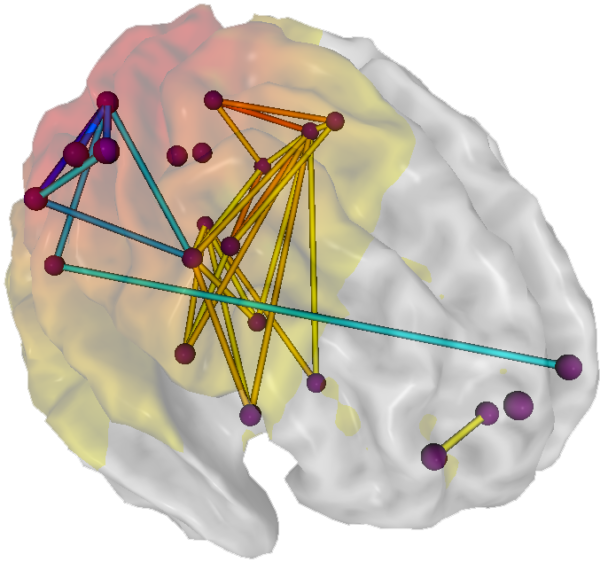
19-channel qEEG and swLORETA methods are not universally available, however. Nevertheless, network findings also suggest that one- or two-channel neurofeedback can also helpfully be directed to individual nodes and pairs of nodes and their connectivity implicated in particular conditions. For example, in a person suffering from depression, one- or two-channel neurofeedback could address dysrhythmias at easily accessible network nodes identified by Li et al. (2018), such as Fp1/Fp2, F3/F4, and Pz. Beneficial results from training at Fp1 and F3 for depression documented by Hammond (2005) provide convergent validation for considering such an approach. A neurofeedback practitioner with the capability to conduct one- or two-channel training could, as Hammond did, uptrain beta frequencies in the left prefrontal cortex to address symptoms of depressed mood.
Similarly with GAD, identifying abnormal functioning in cortical sites and their connections via qEEG assessment, with subsequent swLORETA training, can focus on network findings in GAD by Li et al. (2020). The networks of importance for GAD also involve two 10-20 sites that might be considered for one- or two-channel amplitude or connectivity training: Fz (superior frontal gyrus) and T3/T4 (insula). Hosseini et al. (2016) present a study investigating neurofeedback for military personnel with GAD. Salama et al. (2022) studied GAD in children and adolescents, finding elevated high beta in central and midline cortex regions. One-channel neurofeedback was used to reward alpha and SMR or to inhibit high beta at sites around the vertex, depending on individual-specific qEEG results. Results showed outcomes of equal efficacy to those of CBT. Thus, a neurofeedback practitioner with one- or two-channel methods available could consider the convergent evidence of Li et al. (2020) and Salama et al. (2022) to choose midline sites such as Fz and Cz to uptrain frequencies between 8 and 15 Hz and downtrain frequencies in the high beta range for GAD.
Network analyses in PTSD show reduced connectivity in the default mode network (DMN) (Ross and Cisler, 2020). Practitioners with access to qEEG assessment and 19-channel training software could, therefore, consider individualized connectivity training for the default mode network in their clients with PTSD if findings out of the norm for the DMN are seen in qEEG assessment results. Using one-channel training, however, Nicholson et al. (2023) conducted a randomized sham-controlled study of alpha downtraining at Pz for PTSD with very promising results based on Pz's proximity to the default mode network's hub in the posterior cingulate. Therefore, providers with one- or two-channel equipment could also consider selecting Pz as a site at which to downtrain alpha for their PTSD clients based on the convergent evidence from Ross and Cisler (2020) and Nicholson et al. (2023).
With ADHD, connectivity anomalies were found by Luo et al. (2023) in the default mode network (DMN), sensorimotor network, and fronto-parietal networks, depending on the ADHD symptom cluster. If qEEG findings show results that are out of the norm for a network identified for a symptom cluster seen in a client, 19-channel swLORETA neurofeedback could be considered for neurofeedback with that network. Absent swLORETA equipment, one- or two-channel training along the central strip or at frontal sites could be chosen for neurofeedback consistent with Luo et al.'s (2023) report and neurofeedback research cited by Enriquez-Geppert et al. (2023).
Conclusion
Scientific and clinical understanding of the brain and its function has developed far beyond earlier times when it was considered to work as a homogeneous organ. Its function is now analyzed from micro to macro scales, with attention given to how the activity of collections of locally-situated neurons and their associated glial and vascular partners do their work in conjunction with other larger or smaller regions of interest in connected networks that trade off with one another based on exploratory, homeostatic, and allostatic needs.
As basic brain science has progressed, its methods and findings have been extended to explore whether those who experience various diagnostic conditions demonstrate dysrhythmias in candidate networks. These efforts have been productive.
Network analyses imply that neurofeedback, peripheral biofeedback, and other treatment modalities (e.g., medicine, cognitive behavior therapy) can be helpful allies whose concurrent or sequential delivery may result in better and more durable client outcomes.
This post highlights examples of structural and functional brain networks, anomalies in those networks seen in several diagnostic groups, and how those findings can be integrated with client presentation and scientific findings from studies of neurofeedback to collaborate with clients to choose neurofeedback protocols most likely to benefit the client. An important conclusion is that neurofeedback practitioners should consider the intersection of these areas (i.e., basic brain network science, network studies in diagnostic groups, neurofeedback efficacy research, available training methods, client conditions, client preference) when choosing options to consider for providing neurofeedback. Based on the intersection of these considerations, the provider is then in a strong position to ethically decide with their clients which neurofeedback protocol is most likely to help meet training goals so that the client can most flexibly engage their physiology and behavior for healthy living.
Glossary
ADHD: a neurodevelopmental disorder characterized by inattention, hyperactivity, and impulsivity. agraphia: the loss of the ability to write, typically due to brain damage or a neurological disorder.
alexia: a condition where an individual has difficulty with lexical processing, including reading and recognizing words.
alexia without agraphia: inability to read while retaining the ability to write.
allostasis: the process of achieving stability through physiological or behavioral changes.
alpha power: the amplitude of the EEG frequency band associated with relaxed wakefulness.
anterior cingulate cortex (ACC): a brain region involved in emotional processing and regulating autonomic functions.
anterior insula: a brain region involved in emotional awareness and interception.
arcuate fasciculus: a white matter tract connecting language processing areas in the brain.
basal ganglia: structures involved in motor control, procedural learning, and emotion regulation.
brain network: interconnected regions of the brain working together to perform functions.
brain network directionality: the flow of information between brain regions within a network.
Broca's area: a brain region critical for speech production and language processing.
Brodmann areas: cortical regions defined by distinct cytoarchitectural characteristics.
canonical networks: fundamental, consistent networks within the brain that are involved in basic cognitive processes, such as the default mode network or the salience network.
Carl Wernicke: neurologist who identified the brain area for language comprehension.
central executive network: a network involved in high-level cognitive processes like attention and working memory.
central nervous system (CNS): the brain and spinal cord, controlling most bodily functions.
clusters: groups of neurons or brain regions with coordinated activity.
cytoarchitectonic: the cellular composition and organization of the brain's cortex, used to define distinct regions based on histological features.
default mode network (DMN): a network active during rest and mind-wandering, involved in self-referential thoughts.
delta power: the amplitude of the EEG frequency band associated with deep sleep and slow-wave sleep.
dorsolateral prefrontal cortex (DLPFC): a cortical region involved in executive functions like planning, reasoning, and working memory.
dorsal anterior cingulate: a subregion of the anterior cingulate cortex involved in cognitive and emotional regulation.
dorsal attentional network: a brain network involved in top-down attention control and goal-directed actions.
downtrain: reducing activity in specific brain regions or frequencies, often in neurofeedback.
dysphasia: a condition affecting the ability to communicate due to brain injury.
dysrhythmias of cortical sites and networks: abnormal electrical activity patterns in brain regions.
dynamic functional connectivity: the time-varying interactions between brain regions during different states.
electrocorticography (ECoG): measuring cortical electrical activity using electrodes placed on the brain's surface.
enteric nervous system (ENS): the neural network in the gastrointestinal tract that controls digestion.
executive functions: higher-order cognitive processes, including planning, decision-making, and inhibitory control.
fasciculus: a bundle of nerve fibers (axons) that connect different regions of the brain or spinal cord.
Franz Gall: the founder of phrenology, which associated brain regions with specific mental functions.
functional connectivity: the temporal correlation between spatially remote neurophysiological events.
functional magnetic resonance imaging (fMRI): an imaging technique measuring brain activity by detecting changes in blood flow and metabolism.
functional networks: brain networks defined by synchronized activity across regions during specific tasks or states.
gut-brain coupling: the bidirectional communication between the gut microbiota and the brain.
gyrus: a ridge or fold on the cerebral cortex, surrounded by one or more sulci or fissures.
heart rate variability (HRV) biofeedback: a technique to improve HRV through controlled breathing and relaxation exercises.
homeostasis: the process by which the body maintains a stable internal environment despite changes in external conditions.
hubs: central nodes in a network that connect multiple other nodes.
intrinsic cardiac nervous system: the heart's own neural network, influencing cardiac function.
intrinsic connectivity networks (ICNs): networks identified by consistent patterns of functional connectivity at rest.
irritable bowel syndrome (IBS): a gastrointestinal disorder characterized by chronic abdominal pain and altered bowel habits.
left hemifield neglect: a condition in which a person fails to attend to stimuli on their left side, often due to right hemisphere damage.
left perisylvian pathway: a neural pathway involved in language processing.
limbic network: a network involved in emotion regulation, memory, and motivation.
magnetic resonance imaging (MRI): an imaging technique using magnetic fields and radio waves to create detailed images of the body's internal structures.
Major Depressive Disorder (MDD): a mental disorder characterized by persistent feelings of sadness and loss of interest.
magnetoencephalography (MEG): a technique for mapping brain activity by recording magnetic fields produced by neuronal activity.
microbiome tissue system: the collective microbiota residing in the body, influencing health and disease.
nerve: A bundle of axons in the peripheral nervous system that transmits sensory or motor information to and from the central nervous system.
neurofeedback amplitude training: a technique that trains individuals to regulate the amplitude of specific brain waves.
neurofeedback connectivity training: a method focusing on training the connectivity between different brain regions.
nodes: discrete brain regions that are part of a network.
orbitofrontal cortex (OFC): a prefrontal cortex region involved in decision-making and reward processing.
pars triangularis: a subregion of Broca's Area involved in language production.
Paul Broca: a neurologist who discovered the brain area responsible for speech production.
phrenology: an outdated theory linking skull shape to mental abilities and personality traits.
positron emission tomography (PET) imaging: a functional imaging technique measuring metabolic processes.
randomized sham-controlled study: an experimental design used to test the efficacy of an intervention by comparing it with a credible but inert condition.
regions of interest (ROIs): specific areas of the brain selected for focused analysis in neuroimaging studies.
resting state networks: brain networks identified by correlated activity patterns during rest.
right perisylvian pathway: a neural pathway involved in spatial perception and the communication and understanding of meaning based on modulation of speech sounds (prosody).
salience network: a network that detects and filters important stimuli, aiding in shifting attention.
sensorimotor network: a network involved in sensory processing and motor control.
splenium of the corpus callosum: the posterior part of the corpus callosum, connecting the brain's hemispheres.
structural connectivity: the anatomical connections between brain regions.
structural networks: networks based on the physical connections between brain regions.
superior temporal gyrus: a region involved in auditory processing and language comprehension.
standardized weighted Low-Resolution Electromagnetic Tomography (swLORETA): a method for estimating the location of brain activity.
sulcus: a groove or furrow on the brain's surface, separating adjacent gyri.
sympathetic activity: part of the autonomic nervous system that prepares the body for fight-or-flight responses.
thalamus: a brain structure that relays sensory and motor signals to the cerebral cortex.
ventral attentional network: a network involved in bottom-up detection of unexpected stimuli and reorienting attention.
ventromedial prefrontal cortex: a brain region involved in emotional regulation and decision-making.
visual network: a network involved in processing visual information.
Wernicke's Area: a brain region involved in language comprehension.
References
Alves, P. N., Foulon, C., Karolis, V., Bzdok, D., Margulies, D. S., Volle, E., & Thiebaut de Schotten, M. (2019). An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Communications Biology, 2, 370. https://doi.org/10.1038/s42003-019-0611-3
Baker, C. M., Burks, J. D., Briggs, R. G., Conner, A. K., Glenn, C. A., Morgan, J. P., Stafford, J., Sali, G., McCoy, T. M., Battiste, J. D., O'Donoghue, D. L., & Sughrue, M. E. (2018). A connectomic atlas of the human cerebrum-Chapter 2: The lateral frontal lobe. Operative neurosurgery, 15(suppl_1), S10–S74. https://doi.org/10.1093/ons/opy254
Bañuelos, C., & Ph.D., T. V. (2019). How structural brain network topologies associate with cognitive abilities in a value-based decision-making task. IMPULSE, 16(1). https://impulse.pubpub.org/pub/g0g05m8w
Barrett, L. F. (2020). Seven and a half lessons about the brain. Houghton Mifflin Harcourt.
Bem Junior, L. S., Lemos, N. B., de Lima, L. F. G., Dias, A. J. A., Neto, O. D. C. F., de Lira, C. C. S., Diniz, A. M. S., Rabelo, N. N., Barroso, L. K. V., Valença, M. M., & de Azevedo Filho, H. R. C. (2021). The anatomy of the brain - learned over the centuries. Surgical Neurology International, 12, 319. https://doi.org/10.25259/SNI_200_2021
Boerger, T. F., Pahapill, P., Butts, A. M., Arocho-Quinones, E., Raghavan, M., & Krucoff, M. O. (2023). Large-scale brain networks and intra-axial tumor surgery: A narrative review of functional mapping techniques, critical needs, and scientific opportunities. Frontiers in Human Neuroscience, 17, 1170419. https://doi.org/10.3389/fnhum.2023.1170419
Braun, U., Harneit, A., Pergola, G., Menara, T., Schäfer, A., Betzel, R. F., Zang, Z., Schweiger, J. I., Zhang, X., Schwarz, K., Chen, J., Blasi, G., Bertolino, A., Durstewitz, D., Pasqualetti, F., Schwarz, E., Meyer-Lindenberg, A., Bassett, D. S., & Tost, H. (2021). Brain network dynamics during working memory are modulated by dopamine and diminished in schizophrenia. Nature Communications, 12(1), 3478. https://doi.org/10.1038/s41467-021-23694-9
Brodmann, K. (2006). Brodmann's localization in the cerebral cortex. Springer. https://doi.org/10.1007/b138298
Bullmore, E., & Vértes, P. (2013). From Lichtheim to rich club: Brain networks and psychiatry. Journal of the American Medical Association: Psychiatry, 70, 780–782. https://doi.org/10.1001/jamapsychiatry.2013.212
Buzsáki, G. (2019). Brain from inside out. Oxford University Press. https://doi.org//10.1093/oso/9780190905385.001.0001
Catani, M., & Thiebaut de Schotten, M. (2012). Atlas of human brain connections. Oxford University Press. https://doi.org//10.1093/med/9780199541164.001.0001
Cheema, I., & Chen, T. (2020). Alexia without agraphia as a manifestation of posterior reversible encephalopathy syndrome. Canadian Journal of Neurological Sciences, 48, 1-9. https://doi.org/10.1017/cjn.2020.251
Corcoran, A. W., Perrykkad, K., Feuerriegel, D., & Robinson, J. E. (2023). Body as first teacher: The role of rhythmic visceral dynamics in early cognitive development. Perspectives on Psychological Science, 17456916231185343.
Enriquez-Geppert, S., Brown, T., Heinrich, H., Arns, M., & Garcia Pimenta, M. Attention deficit hyperactivity disorder (ADHD). In I. Khazan, F. Shaffer, D. Moss, R. R. Lyle, and S. Rosenthal (Eds.), Evidence-based practice in biofeedback and neurofeedback (4th ed.) (121-141). Aurora, CO: Association for Applied Psychophysiology and Biofeedback.
Finger S. (2009). The birth of localization theory. In M. J. Aminoff, F. Boller, & D. F. Swaab (Eds.), Handbook of clinical neurology, 95 (pp. 117-128). Elsevier. https://doi.org/10.1016/S0072-9752(08)02110-6. PMID: 19892113.
Ganzel, B. L., Morris, P. A., & Wethington, E. (2010). Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychological Review, 117, 134-74. https://doi.org/10.1037/a001777
Hagmann, P., Cammoun, L., Gigandet, X., Meuli, R., Honey, C. J., Wedeen, V. J., & Sporns, O. (2008). Mapping the structural core of human cerebral cortex. PLoS Biology, 6(7), e159. https://doi.org/10.1371/journal.pbio.0060159
Hearne, L., Mattingley, J., & Cocchi, L. (2016). Functional brain networks related to individual differences in human intelligence at rest. Scientific Reports, 6, 32328. https://doi.org//10.1038/srep32328
Heinonen, J., Numminen, J., Hlushchuk, Y., Antell, H., Taatila, V., & Suomala, J. (2016). Default mode and executive network areas: Association with the serial order in divergent thinking. Public Library of Science, 11(9), e0162234.
https://doi.org/10.1371/journal.pone.0162234
Hutchison, R. M., Womelsdorf, T., Allen, E. A., Bandettini, P. A., Calhoun, V. D., Corbetta, M., Della Penna, S., Duyn, J. H., Glover, G. H., Gonzalez-Castillo, J., Handwerker, D. A., Keilholz, S., Kiviniemi, V., Leopold, D. A., de Pasquale, F., Sporns, O., Walter, M., & Chang, C. (2013). Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage, 80, 360–378. https://doi.org/10.1016/j.neuroimage.2013.05.079
Jacobs, K. M. (2018). Brodmann's areas of the cortex. In J. S. Kreutzer, J. DeLuca, & B. Caplan, B. (Eds.), Encyclopedia of clinical neuropsychology (p. 647). Springer.
Laird, A. R., Fox, P. M., Eickhoff, S. B., Turner, J. A., Ray, K. L., McKay, D. R., Glahn, D. C., Beckmann, C. F., Smith, S. M., & Fox, P. T. (2011). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience, 23(12), 4022–4037. https://doi.org/10.1162/jocn_a_00077
Lashley, K. S. (1930). Basic neural mechanisms in behavior. Psychological Review, 37, 1–24. https://doi.org/10.1037/h0074134
Li, B. J., Friston, K., Mody, M., Wang, H. N., Lu, H. B., & Hu, D. W. (2018). A brain network model for depression: From symptom understanding to disease intervention. CNS Neuroscience & Therapeutics, 24, 1004-1019. https://doi.org/10.1111/cns.12998
Li, J., Liu, Y., Wisnowski, J. L., & Leahy, R. M. (2023). Identification of overlapping and interacting networks reveals intrinsic spatiotemporal organization of the human brain.
NeuroImage, 270. https://doi.org//10.1016/j.neuroimage.2023.119944
Llinás, R. R. (2001). I of the vortex: From neurons to self. MIT Press. https://doi.org//10.7551/mitpress/3626.001.0001
Luo, L., Chen, L., Wang, Y., Li, Q., He, N., Li, Y., You, W., Wang, Y., Long, F., Guo, L., Luo, K., Sweeney, J. A., Gong, Q., & Li, F. (2023). Patterns of brain dynamic functional connectivity are linked with attention-deficit/hyperactivity disorder-related behavioral and cognitive dimensions. Psychological Medicine, 53(14), 1–12. Advance online publication. https://doi.org/10.1017/S0033291723000089
Mayer, E. A., Ryu, H. J., & Bhatt, R. R. (2023). The neurobiology of irritable bowel syndrome. Molecular Psychiatry, 28, 1451-1465. https://doi.org/10.1038/s41380-023-01972-w
Medaglia, J. D. (2019). Networks of cognitive processes: Functional and anatomical correlates of cognition, emotions, and social cognition. In B. T. Baune & C. Harmer (Eds.), Cognitive dimensions of major depressive disorder (pp. 157–170). Oxford University Press. https://doi.org/10.1093/med/9780198810940.003.0013
Menon, V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Science,15, 483-506. https://doi.org/10.1016/j.tics.2011.08.003
Menon, V., & Uddin, L.Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function, 214, 655–667.
https://doi.org/10.1007/s00429-010-0262-0
Nicholson, A. A., Densmore, M., Frewen, P. A., Neufeld, R. W. J., Théberge, J., Jetly, R., Lanius, R. A., & Ros. T. (2023). Homeostatic normalization of alpha brain rhythms within the default-mode network and reduced symptoms in post-traumatic stress disorder following a randomized controlled trial of electroencephalogram neurofeedback. Brain Communications, 5(2):fcad068. https://doi.org/10.1093/braincomms/fcad068
Pessoa, L. (2014). Understanding brain networks and brain organization. Physics of Life Review, 11, 400-435. https://doi.org/10.1016/j.plrev.2014.03.005
Porcelli, S., Van Der Wee, N., van der Werff, S., Aghajani, M., Glennon, J. C., van Heukelum, S., Mogavero, F., Lobo, A., Olivera, F. J., Lobo, E., Posadas, M., Dukart, J., Kozak, R., Arce, E., Ikram, A., Vorstman, J., Bilderbeck, A., Saris, I., Kas, M. J., & Serretti, A. (2019). Social brain, social dysfunction and social withdrawal. Neuroscience and Biobehavioral Reviews, 97, 10–33. https://doi.org/10.1016/j.neubiorev.2018.09.012
Raichle, M. E. (2015). The brain's default mode network. Annual Review of Neuroscience, 38, 433-447. https://doi.org/10.1146/annurev-neuro-071013-014030
Ross, M. C., & Cisler, J. M. (2020). Altered large-scale functional brain organization in posttraumatic stress disorder: A comprehensive review of univariate and network-level neurocircuitry models of PTSD. NeuroImage: Clinical, 27, 102319.
https://doi.org/10.1016/j.nicl.2020.102319
Ryyppö, E, Glerean, E., Brattico, E., Saramäki, J., & Korhonen, O. (2018). Regions of Interest as nodes of dynamic functional brain networks. Network Neuroscience, 2, 513–535. https://doi.org/10.1162/netn_a_00047
Salama, A. M., Abdel-Latif, S., Omar, T., & El Wafa, H. A. (2022). Neurofeedback training and cognitive behavior therapy for treatment of generalized anxiety disorders in children and adolescents: A comparative study. NeuroRegulation, 9, 28-39. https://doi.org/10.15540/nr.9.1.29
Schimmelpfennig, J., Topczewski, J., Zajkowski, W., & Jankowiak-Siuda, K. (2023). The role of the salience network in cognitive and affective deficits. Frontiers in Human Neuroscience, 17:1133367. https://doi.org/10.3389/fnhum.2023.1133367
Seitzman, B. A., Snyder, A. Z., Leuthardt, E. G., & Shimony, J. S. (2019). The state of resting state networks. Topics in Magnetic Resonance Imaging, 28, 189-196. https://doi.org/ 10.1097/RMR.0000000000000214
Sridharan, D., Levitin, D. J., & Menon, V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences, 105, 12569–12574. https://doi.org/10.1073/pnas.0800005105
Sterling, P. (2014). Homeostasis vs allostasis: Implications for brain function and mental disorders. Journal of the American Medical Association: Psychiatry, 71, 1192–1193. https://doi.org/10.1001/jamapsychiatry.2014.1043
Strozer, M. (2009). One Century of brain mapping using Brodmann Areas. Clinical Neuroradiology, 19, 179-186.
Tekin, S., & Cummings, J. L. (2002). Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. Journal of Psychosomatic Research, 53, 647-654. https://doi.org/10.1016/s0022-3999(02)00428-2
Uddin, L. Q., Yeo, B. T. T., & Spreng, R. N. (2019). Towards a universal taxonomy of macro-scale functional human brain networks. Brain Topography, 32, 926–942. https://doi.org//10.1007/s10548-019-00744-6
Vieito, J., Pownall, R, & Rocha, A., Rocha, F., & Massad, E. (2015). The Neural behavior of investors. Paper presented at the meeting of the American Economic Association ASSA, Boston.
Yao, Z., Hu, B., Xie, Y., Moore, P., & Zheng, J. (2015). A review of structural and functional brain networks: small world and atlas. Brain Informatics, 2(1), 45–52. https://doi.org/10.1007/s40708-015-0009-z
Yeo, B. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., Roffman, J. L., Smoller, J. W., Zöllei, L., Polimeni, J. R., Fischl, B., Liu, H., & Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. https://doi.org/10.1152/jn.00338.2011
Support Our Friends at NRBS, ISNR, and AAPB








Very good! Thank you.