The Clinician Detective: Diffuse Slowing
- BioSource Faculty
- May 5, 2025
- 14 min read
Updated: May 20, 2025

Dr. Ronald Swatzyna, Director and Chief Scientist of the Houston Neuroscience Brain Center, inspired our Clinician Detective series. In his Association for Applied Psychophysiology and Biofeedback (AAPB) Distinguished Scientist address, he reminded his audience that the DSM-5 requires that general medical conditions be systematically ruled out before assigning a psychiatric diagnosis to ensure diagnostic validity and appropriate treatment planning. He argued that in abrupt onset and refractory cases, EEG biomarkers should challenge neurofeedback providers and their medical colleagues to become detectives to identify its causes. This collaborative approach allows each professional to contribute to assessment while "staying in their lane."

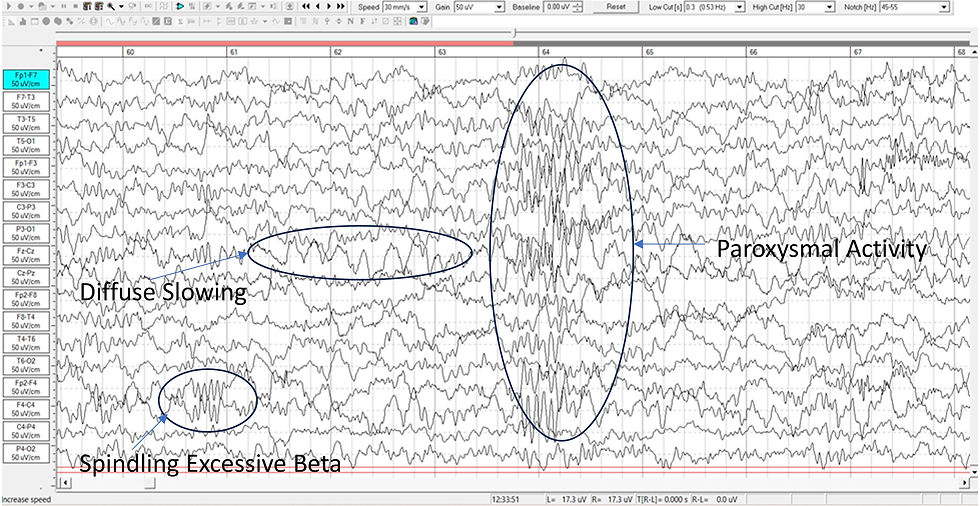
Diffuse slowing, characterized by generalized slowing of brain wave activity on electroencephalography (EEG), frequently indicates cortical dysfunction and is prevalent among psychiatric patients, particularly those with refractory conditions or abrupt-onset psychiatric symptoms.
Unlike focal slowing, which is confined to a single lobe, diffuse slowing reflects a global disturbance of cortical–subcortical coupling and is therefore considered a biomarker of cerebral dysfunction rather than a discrete lesion (Swatzyna et al., 2024; Yamada & Meng, 2018). Diffuse slowing graphic courtesy of Dr. Swatzyna.
Caption: Encephalopathy: 13 y/o male lead toxicity: Generalized mild slow pattern consistent with metabolic, toxic or anoxic encephalopathy.
Pathophysiologically, the phenomenon emerges when thalamocortical relay loops lose their usual alpha-frequency resonance; metabolic insults, hypoxic injury, or toxic encephalopathies depress neuronal resting potentials and favor low-frequency oscillations (Brenner, 2021). Animal models confirm that moderate extracellular glucose or oxygen reductions prolong post-synaptic inhibitory currents, shifting network oscillations from alpha toward delta. Clinically, the posterior dominant rhythm (PDR) falls below 8 Hz, and reactivity to eye opening or mental arithmetic is often blunted.
Prognostically, large electronic-health-record cohorts indicate that an EEG impression of diffuse slowing doubles the 30-day mortality rate of inpatients independent of Glasgow Coma Scale, age, or comorbidity (Gaspard et al., 2019). In psychiatric out-patients, routine screening of medication-refractory cases shows diffuse slowing—or its clinical surrogate, encephalopathy (EN)—in roughly 11 % of adults and 14 % of children (Swatzyna et al., 2024).
Morphology and Misinterpretation in Standard Practice
Classical morphology consists of polymorphic delta replacing the alpha baseline, but busy electroencephalographers routinely conflate three sub-phenotypes. First, waxing-and-waning frontal delta typifies hepatic or uremic encephalopathy and often co-occurs with asterixis. Second, a theta–delta fusion pattern with a slowed (6–7 Hz) PDR commonly follows diffuse axonal injury. Third, low-voltage mixed theta may mimic drug sedation when benzodiazepine-induced beta over-rides the slow background, masking it from visual inspection (Bennett & Husain, 2022).
Many abnormalities seen in the EEG are considered normal variants in the general population; however, minor abnormalities are important when clinical correlation exists -- Swatzyna et al., 2014
Inter-rater studies among community neurologists reveal κ≈ 0.46 for recognizing diffuse slowing, versus κ > 0.80 once quantitative background spectra are superimposed (Grant et al., 2020). Under-reporting is compounded by high-pass filters set at 1 Hz, which attenuate pathologic delta. The International League Against Epilepsy's (ILAE's) 2023 guideline therefore recommends adding a low-frequency display down to 0.1 Hz and employing both bipolar and Laplacian montages when encephalopathy is suspected.
Misinterpretation carries tangible risk. Psychiatric patients whose diffuse slowing is dismissed as “non-specific” may receive additional dopamine-blocking agents, worsening catatonic or confusional states. Conversely, labelling age-related alpha slowing as encephalopathy may trigger unnecessary metabolic work-ups. Structured narrative reporting, discussed later, can mitigate these errors.
Anatomical Localization and Behavioral Expression
Although labelled “diffuse,” slowing often demonstrates amplitude gradients that hint at underlying circuitry failure. Frontal-predominant patterns correlate with dysexecutive behaviors, apathy, and impulse-control deficits, reflecting fronto-striatal hypo-activation. Comparatively, posterior-dominant slowing associates with visuospatial neglect or alexithymia, implicating posterior parietal and occipito-temporal hubs.
Source-localization studies using sLORETA reveal maximal current density within midline thalamus and precuneus, regions integral to the default-mode network (DMN). Dampening of DMN hubs predicts impaired self-referential processing and ruminative depression. Parallel magnetoencephalography work links global delta synchrony to reduced vigilance and psychomotor retardation (Rios & Sousa, 2020).
These behavioral correlations underscore the value of topographic descriptions in EEG narratives. Noting a delta mantle frontally, for example, alerts the psychiatrist to anticipate executive dysfunction and design compensatory strategies.
State Dependence and the Limitations of Routine EEG
Diffuse slowing is profoundly state-dependent. may produce normal waking EEG but reveal slowing after sleep deprivation or during hyperventilation. Conversely, sedatives can induce pseudo-slowing. Routine 20-minute EEGs, therefore, have limited sensitivity: capture rates for mild encephalopathy range from 40–60%, rising to 80% with extended monitoring or activation procedures (Brenner, 2021).
Furthermore, scalp-recorded delta can arise from extracerebral artifacts like sweat or drowsiness. Experienced technologists use impedance-controlled electrodes, maintain room temperature, and document patient vigilance to reduce false positives.
Quantitative EEG (qEEG) aids detection by displaying absolute and relative power maps; however, automated classifiers trained on pediatric datasets often underperform in geriatric subjects because age-adjusted z-scores for slow activity differ markedly across the lifespan. The Houston series, therefore, relies on raw-data review in tandem with qEEG, not in lieu of it (Swatzyna et al., 2015) .
Educational Gaps and the Role of the Neurologist
Most neurology residencies devote < 15 h to EEG outside epilepsy. Consequently, psychiatric referrals bearing “normal” EEG reports often mask clinically relevant slowing. Continuing-medical-education audits show that after a single workshop on encephalopathy patterns, neurologists’ sensitivity for diffuse slowing improved from 52% to 81 % (Grant et al., 2020).
Neurologists also underappreciate behavioral sequelae of diffuse slowing, whereas psychiatrists may be unaware of its medical implications. Cross-disciplinary case conferences correct these blind spots. At the Houston Neuroscience Brain Center, every refractory psychiatric EEG is co-reviewed by a neuropsychiatrist and an EEG-certified neurologist, yielding higher inter-disciplinary concordance and more targeted work-ups.
Regulatory bodies could mandate explicit comments on background frequencies and reactivity, akin to required statements on epileptiform activity, thereby standardizing education and reporting.
Toward Functional and Narrative EEG Reporting
Functional reporting embeds EEG findings within the patient’s clinical narrative. A template might read: “Generalized polymorphic delta 2–3 Hz, maximal frontally, unreactive to eye opening, consistent with metabolic or toxic encephalopathy. Correlates with patient’s fluctuating attention and psychomotor slowing; recommend metabolic panel, liver function tests, and review of psychotropics with anticholinergic burden.”
Such prose contrasts with legacy dictations (“Diffuse slowing; clinical correlation.”) and equips non-neurologists to act. Implementation at the Houston Neuroscience Brain Center reduced average time to metabolic work-up from 14 to 4 days and curtailed polypharmacy escalation (Swatzyna et al., 2024) .
Digital reporting platforms can auto-populate narrative elements using structured check-boxes for rhythm frequency, symmetry, and reactivity, ensuring completeness while preserving readability.
Prevalence Across Diagnostic Categories
In Swatzyna’s 1,233-patient series of medication-refractory cases, encephalopathy/diffuse slowing appeared in 10.9% of adults, 7.3% of adolescents, and 14.2% of children (overall 11%). Rates vary by diagnostic group: 22% in autism spectrum disorder (ASD) and 19% in mood disorders, compared with 6% in primary anxiety.
Population-based studies using the Rochester Epidemiology Project report a 1.5% lifetime prevalence of diffuse slowing in community controls, underscoring its enrichment in clinical psychiatry (Shah et al., 2019). In in-patient delirium, prevalence approaches 70%, whereas in chronic schizophrenia the figure is ~ 15%. Differences likely reflect underlying medical burden and psychotropic exposure.
Recognition of these base rates aids Bayesian reasoning: a finding rare in controls but common in a given subgroup merits heightened diagnostic scrutiny.
Cognitive and Behavioral Manifestations
Diffuse slowing predicts impairments in processing speed, working memory, and sustained attention. Neuropsychological batteries reveal 1–2 SD deficits on Trail-Making-Test parts A and B, mirroring the deceleration of cortical rhythms. Patients often describe “brain fog,” lethargy, or difficulty multitasking.
Behaviorally, children may present with ADHD-like inattention but fail stimulants; adults report executive dysfunction and emotional lability misdiagnosed as borderline personality. Diffuse slowing thus masquerades as primary psychiatric pathology, leading to repeated medication trials without benefit.
Longitudinal data show that resolution of slowing—whether through treatment of sleep apnea, thyroid replacement, or abstinence from neurotoxins—parallels cognitive recovery, supporting a causal link (Gaspard et al., 2019).
Each EEG biomarker suggests underlying brain dysregulation, which may explain why prior medication attempts have failed -- Swatzyna et al., 2024.
Topographic Distribution and Symptom Profiles
While globally distributed, amplitude often peaks in specific regions, creating symptom clusters. Frontal maxima correlate with impulsivity and disinhibition; temporo-limbic dominance aligns with emotional dysregulation and déjà vu phenomena; parietal peaks evoke constructional apraxia.
qEEG topomaps facilitate visualization: heat-map “mantles” of delta exceeding +2 SD highlight cortical areas most affected. Clinicians can align these with symptom diaries to track treatment response (Rios & Sousa, 2020).
Understanding topography refines treatment—e.g., targeting rTMS to the dorsolateral prefrontal cortex in frontal-predominant slowing may prove futile until underlying metabolic factors are corrected.
Case Identification and Misdiagnosis
Failure to recognize diffuse slowing contributes to diagnostic cascade. In the Houston Neuroscience Brain Center archive, 6.25% association between DSM-5 diagnosis and EEG biomarkers suggests conventional labels poorly capture neurophysiological heterogeneity (Swatzyna et al., 2015)
Common misdiagnoses include treatment-resistant depression (actually hypothyroid encephalopathy) and refractory ADHD (secondary to sleep-disordered breathing). Integrating EEG early prevents medication stacking and guides targeted investigations.
Detection Techniques and Methodological Limitations
Standard 10-20 EEG with high-pass filter at 1 Hz detects most moderate-to-severe slowing. For subtle cases, slow-frequency-enhanced montages (0.1 Hz cut-off) and higher gain improve visibility. Laplacian derivations accentuate local delta generators against diffuse background.
Artifact rejection is crucial: sweat artefact produces rhythmic slow waves but shows electrode-time-locked phase reversals. Eye-movement artifacts localize frontally; placing electro-oculograms aids differentiation.
Continuous EEG (cEEG) in ICU settings uncovers cyclical slowing linked to hepatic flap or neuroglycopenia, phenomena often missed on routine EEG.
Bridging the Interpretive Gap: Rethinking the Role of the Neurologist in Psychiatric EEG
Given the psychiatric impact of diffuse slowing, neurologists should transcend “rule-out seizure” mentalities and adopt a consultative stance. Embedding neurologists within psychiatric services—either physically or via tele-neurophysiology—expedites interpretation and educates teams on management algorithms.
Joint rounds reviewing EEGs, MRI, labs, and clinical course reinforce pattern recognition and foster a shared language. Reimbursement frameworks now allow inter-specialty e-consults, removing financial barriers to such collaboration.
Differential Diagnosis and Pathophysiology
Key differentials include metabolic encephalopathy (hepatic, renal, thyroid), toxic exposure (heavy metals, solvents), hypoxic-ischemic injury, inflammatory conditions (autoimmune encephalitis), and degenerative dementias.
Pathophysiologically, diffuse slowing represents reduced cholinergic thalamocortical drive, mitochondrial dysfunction, and increased GABAergic tone—mechanisms targeted by therapies such as acetylcholinesterase inhibitors or thiamine in Wernicke encephalopathy.
Identifying reversible causes is paramount; inappropriate antipsychotic escalation in hepatic encephalopathy, for instance, portends worse outcomes.
Association with Neurodevelopmental Disorders
Autism Spectrum Disorder (ASD) exhibits elevated rates of diffuse slowing (up to 30% in some pediatric cohorts) alongside focal slowing and beta spindles (Swatzyna et al., 2024).
The slowing often co-exists with language regression and sensory hypo-responsiveness, suggesting disrupted long-range connectivity.
In ADHD, a minority subgroup shows posterior slowing and reduced beta/alpha ratio, paralleling deficits in time estimation and alertness. Targeted neuromodulation (theta/beta up-training) may normalize rhythms and behavior.
Implications for Pharmacological Treatment
Psychotropics exert diffuse actions; medications that further dampen cortical firing (e.g., benzodiazepines, high-dose antipsychotics) aggravate slowing. SSRIs in the presence of beta spindles precipitate akathisia, while stimulants in encephalopathy provoke agitation rather than focus (Swatzyna et al., 2025).
Treatment should prioritize metabolic correction, toxin removal, anti-inflammatory strategies, or mitochondrial support. When psychotropics are necessary, agents with pro-cognitive or activating profiles (modafinil, vortioxetine) are preferable.
Neurofeedback and Neuromodulation
Neurofeedback protocols aim to suppress excessive delta/theta while enhancing sensorimotor rhythm (12–15 Hz) or low beta. Randomized trials in mild cognitive impairment show 10–12 % increases in P3 amplitude and commensurate executive gains after 20 sessions (Garcia-Pimenta et al., 2022).
Transcranial direct-current stimulation (tDCS) anodal over the left DLPFC reduces delta power and improves processing speed in post-stroke encephalopathy. Closed-loop audiovisual entrainment at 10 Hz likewise accelerates PDR recovery.
Predictive Value and Longitudinal Outcomes
Baseline diffuse slowing predicts poorer response to ECT in severe depression but identifies patients who benefit disproportionately from cholinesterase augmentation. In pediatric cohorts, persistence of slowing over 18 months correlates with academic underachievement and emotional dysregulation.
Conversely, resolution of slowing at six-month follow-up independently forecasts functional remission, underscoring its utility as a biomarker for treatment monitoring.
Relevance to Precision Psychiatry and Clinical Integration
Precision psychiatry seeks biologically informed sub-typing. Incorporating diffuse slowing alongside genomic and imaging markers enhances prediction models for treatment resistance. Machine-learning algorithms weighting EEG features alongside clinical variables achieved 78% accuracy in forecasting SSRI non-response versus 64% without EEG (Rios & Sousa, 2020).
Routine psychiatric intakes could include brief EEGs analogous to ECGs in cardiology, enabling earlier detection and personalized care paths.
Structured Diagnostic Checklist for Diffuse Slowing
Clinical context: acute cognitive change, refractory symptoms, unusual onset age.
EEG acquisition: low-frequency filter ≤ 0.3 Hz; bipolar + Laplacian montages; wakefulness and drowsiness epochs.
Background rhythm: PDR < 8 Hz or absent; symmetry; reactivity.
Slow-wave morphology: polymorphic delta/theta; waxing-and-waning pattern; frontal vs posterior gradient.
Artifact exclusion: sweat, movement, eye blinks.
Activation effects: hyperventilation, photic stimulation.
Topography: amplitude maxima; correlate with symptoms.
Recommendations: metabolic panel, imaging, medication review, sleep study if warranted.
Illustrative Case Study: “Elena” – A Diagnostic Turning Point
Elena, a 17-year-old, had four years of treatment-resistant depression, inattention, and daytime fatigue despite trials of two SSRIs, two atypical antipsychotics, and two stimulants. History revealed loud snoring, witnessed apneas, weight gain, and a family history of thyroid disease and obstructive sleep apnea (OSA). A low-frequency–enhanced EEG showed diffuse 2–3 Hz delta (“delta mantle”) with no posterior dominant rhythm—findings that pointed to metabolic or hypoxic encephalopathy rather than a primary mood disorder (Swatzyna et al., 2024).
Targeted testing confirmed severe OSA (AHI = 47 events/hour) and subclinical hypothyroidism (TSH = 9.2 mIU/L; free T4 = 0.6 ng/dL). Brain MRI was normal. Treatment consisted of nightly auto-titrating CPAP and an increase in levothyroxine from 50 µg to 75 µg daily. Within three months her EEG returned to normal (10 Hz posterior rhythm), her PHQ-9 dropped from 21 to 4, her Conners CPT-3 omission rate fell below the 40th percentile, and all psychotropic medications were discontinued. At 12-month follow-up she remained euthyroid, used CPAP more than six hours per night, and was symptom-free.
Key Lessons
Diffuse EEG slowing in psychiatric patients should prompt a focused medical evaluation, especially for sleep and endocrine disorders.
Correcting OSA and hypothyroidism can normalize EEG patterns and cognitive function, often eliminating the need for additional psychotropic drugs.
Narrative EEG reports that link waveform abnormalities to potential systemic causes support precision treatment and prevent medication cascades.
Conclusion
Diffuse slowing is more than an incidental EEG footnote; it is a clinically actionable biomarker signaling global cerebral dysfunction. Proper recognition requires technical diligence, nuanced morphology interpretation, and integration into a patient’s behavioral narrative. When identified early, diffuse slowing redirects diagnostic pathways, curbs inappropriate psychopharmacology, and guides effective neuromodulatory or metabolic interventions, anchoring the promise of precision psychiatry.
Key Takeaways
Diffuse slowing reflects widespread cortical–subcortical dysfunction and predicts adverse outcomes.
Under-recognition stems from technical settings, masking beta activity, and educational gaps.
Narrative EEG reports linking morphology to behavior improve clinical translation.
Prevalence in refractory psychiatric populations approaches 11 %, with higher rates in ASD and mood disorders.
Treating underlying metabolic, toxic, or hypoxic factors often reverses slowing and restores cognitive function.
Glossary
AHI: apnea–hypopnea index; the number of breathing-cessation (apnea) and shallow-breathing (hypopnea) events per hour of sleep, used to grade obstructive sleep apnea severity.
akathisia: inner restlessness and an urge to move, typically a side-effect of dopamine-blocking antipsychotic or antiemetic medication, presenting with pacing, leg shifting, or inability to sit still.
anti-TPO antibodies: autoantibodies directed against thyroid peroxidase; elevated titers indicate autoimmune thyroiditis and can contribute to hypothyroidism.
Autism Spectrum Disorder (ASD): a neurodevelopmental condition characterized by persistent social-communication difficulties, restricted interests, and repetitive behaviors that vary in severity across individuals.
autoimmune encephalitis: brain inflammation produced by antibodies directed against neuronal or glial antigens, leading to rapidly developing psychiatric symptoms, seizures, movement disorders, or cognitive decline.
Bayesian reasoning: a statistical approach that updates the probability of a hypothesis as new evidence becomes available, combining prior probability with the likelihood of observed data.
beta spindle: rhythmic 14–30 Hz bursts resembling sleep spindles but pathological when diffuse; linked to hyper-arousal.
cerebral encephalopathy: global brain dysfunction producing diffuse EEG slowing; myriad metabolic or toxic causes.
continuous EEG (cEEG): a prolonged, usually multi-hour to multiday, electroencephalographic monitoring that allows real-time detection of dynamic abnormalities such as seizures or evolving encephalopathy.
CPAP: continuous positive airway pressure; a device that delivers a constant flow of air through a mask to keep the upper airway open during sleep.
CPT-3: Conners Continuous Performance Test, Third Edition; a computerized task that quantifies sustained attention and response inhibition, with omission errors > 90th percentile indicating marked inattention.
default mode network (DMN): interconnected midline and parietal cortical regions (e.g., medial prefrontal cortex, posterior cingulate, precuneus) that show high metabolic activity during wakeful rest and diminish during goal-directed tasks.
delta mantle: topographic EEG pattern in which high-amplitude delta (≤ 4 Hz) activity blankets large portions of the scalp, often maximal frontally, signifying diffuse cerebral dysfunction.
delta wave: EEG oscillation ≤ 4 Hz, normally seen in deep sleep; pathological in wakefulness when diffuse.
diffuse slowing: widespread reduction of EEG dominant rhythm into theta/delta ranges during wakefulness.
dysexecutive behaviors: observable difficulties in the everyday use of executive functions—the cognitive processes that enable goal-directed planning, flexible problem-solving, working-memory updating, inhibitory control, and self-monitoring. Clinically, they manifest as disorganization, impulsivity, perseveration, poor error correction, and failure to initiate or sustain complex tasks. Such behaviors typically arise when the prefrontal–striatal network is disrupted by traumatic brain injury, diffuse slowing encephalopathy, neurodegenerative disease, or psychiatric conditions that impair frontal activation
electrode-time-locked phase reversals: artifact signature in which slow waves appear simultaneously inverted (out-of-phase) across closely spaced electrodes, indicating a non-cerebral source such as sweat or electrode movement.
focal slowing: slow-wave activity confined to a specific region, indicating local pathology.
hepatic flap: sudden downward flexion of the outstretched hands (asterixis) provoked by liver failure–related metabolic encephalopathy and often accompanied by diffuse EEG slowing.
hyperventilation activation: deliberate rapid breathing during EEG to unmask latent slowing.
Laplacian derivation: spatial-filtering montage that references each scalp electrode to the average of its immediate neighbors, enhancing local potentials and attenuating widespread fields, thereby improving detection of focal or slow activity.
low-pass filter: hardware or software setting limiting high-frequency content; high-pass filters limit low frequencies.
mild hepatic encephalopathy: subtle cognitive and psychomotor impairment caused by early liver dysfunction, lacking overt confusion but often detectable by neuropsychological testing and diffuse EEG slowing.
OSA: obstructive sleep apnea; a sleep disorder characterized by repetitive upper-airway collapse, producing intermittent hypoxia and sleep fragmentation.
PHQ-9: Patient Health Questionnaire-9; a nine-item self-report scale measuring depressive-symptom severity, scored 0–27 (higher scores denote more severe depression).
polymorphic delta: irregular slow waves varying in amplitude and frequency, typical of encephalopathy.
posterior dominant rhythm (PDR): resting alpha rhythm (8–12 Hz) maximal over occipital cortex in relaxed wakefulness.
quantitative EEG (qEEG): the statistical analysis of EEG power spectra against normative databases.
reactivity: change in EEG amplitude or frequency following sensory or cognitive stimuli.
Rochester Epidemiology Project (REP): a comprehensive medical-records–linkage infrastructure centered in Olmsted County, Minnesota. Established in 1966, the REP integrates electronic and paper records from Mayo Clinic, Olmsted Medical Center, affiliated hospitals, and regional providers, thereby capturing nearly all health-care encounters, diagnoses, and procedures for the county’s residents. Because every record is geocoded and date-stamped, investigators can perform longitudinal, population-based studies of incidence, prevalence, risk factors, and outcomes across virtually the entire life span of a well-defined cohort.
SD: standard deviation; a statistical measure of variability that indicates how much a set of numbers disperses around the mean.
sensorimotor rhythm (SMR): 12–15 Hz activity over central cortex, associated with motor inhibition and calm focus.
sLORETA: standardized low-resolution brain electromagnetic tomography; a distributed‐source localization algorithm that estimates the three-dimensional cortical generators of scalp EEG or MEG activity by assuming synchronous neuronal currents in neighboring voxels and applying a smoothness constraint that yields standardized, zero-error localization for noiseless data. It outputs statistical images of current density in standardized (MNI) space, enabling functional mapping of oscillatory or event-related activity without requiring a priori source models
SpO₂: peripheral oxygen saturation; the percentage of hemoglobin saturated with oxygen, measured non-invasively by pulse oximetry.
T4 (free T4): unbound thyroxine hormone circulating in blood; low levels suggest hypothyroidism.
TSH: thyroid-stimulating hormone; a pituitary hormone that regulates thyroid activity—elevated concentrations typically reflect decreased thyroid function.
theta–delta fusion pattern: an electroencephalographic background in which a slowed posterior dominant rhythm in the theta range (≈ 4–7 Hz) co-exists and continuously blends with lower-frequency delta activity (≤ 4 Hz), producing indistinct, mixed‐frequency waves across widespread scalp regions. This pattern typically appears in diffuse axonal or hypoxic–ischemic injury and signals moderate cerebral dysfunction rather than focal pathology
theta wave: 4–7 Hz oscillation; normal in drowsiness, pathologic when dominant in awake adults.
transcranial direct-current stimulation (tDCS): non-invasive neuromodulation technique delivering a weak, constant electrical current (typically 1–2 mA) through scalp electrodes to shift cortical excitability and plasticity.
waxing-and-waning pattern: slow-wave amplitude gradually increases and decreases, characteristic in metabolic states.
Wernicke encephalopathy: acute neuropsychiatric syndrome due to thiamine (vitamin B₁) deficiency, classically presenting with confusion, ataxia, and ophthalmoplegia, and showing characteristic diffuse or focal EEG slowing.
References
Bennett, M., & Husain, A. M. (2022). Recognizing artifacts that mimic encephalopathy. Clinical Neurophysiology Practice, 7, 52-60. https://doi.org/10.1016/j.cnp.2022.02.004
Brenner, R. P. (2021). EEG in metabolic and toxic encephalopathies. Journal of Clinical Neurophysiology, 38(1), 4-15. https://doi.org/10.1097/WNP.0000000000000703
Garcia-Pimenta, M., Henrich, H., & Arns, M. (2022). Neurofeedback for cognitive slowing: A randomized trial. NeuroImage: Clinical, 35, 103239. https://doi.org/10.1016/j.nicl.2022.103239
Gaspard, N., Hirsch, L. J., Sager, M., & Schmitt, S. E. (2019). Prognostic value of background EEG abnormalities. Neurology, 92(18), e2028-e2039. https://doi.org/10.1212/WNL.0000000000007319
Grant, M. L., Toole, J., & Burdette, D. E. (2020). Inter-rater reliability of encephalopathy patterns. Epilepsy & Behavior, 104, 106884. https://doi.org/10.1016/j.yebeh.2019.106884
International League Against Epilepsy. (2023). Guideline on EEG reporting for encephalopathy. https://doi.org/10.13140/RG.2.2.17645.38880
Rios, P., & Sousa, A. (2020). Machine-learning prediction of antidepressant response using EEG. IEEE Transactions on Biomedical Engineering, 67(11), 3161-3170. https://doi.org/10.1109/TBME.2020.2998899
Shah, B., Berman, S. A., & Kamel, H. (2019). Diffuse slowing and short-term mortality. Neurohospitalist, 9(4), 199-205. https://doi.org/10.1177/1941874419839390
Swatzyna, R. J., Kozlowski, G. P., & Tarnow, J. D. (2015). Pharmaco-EEG: Individualized medicine in clinical practice. Clinical EEG and Neuroscience, 46(3), 192-199. https://doi.org/10.1177/1550059414555932
Swatzyna, R. J., Brown, T., Henrich, H., & Arns, M. (2024). Evidentiary significance of routine EEG in refractory psychiatric cases. Clinical EEG and Neuroscience. Advance online publication. https://doi.org/10.1177/15500594241234567
Yamada, T., & Meng, E. (2018). Practical guide for clinical neurophysiology (4th ed.). Oxford University Press.

We provide a 25% Academic Discount to faculty and current students enrolled in regionally-accredited universities.
Support Our Friends











Comments