The Clinician Detective: How Did A FOXP4 Variant and A Sellar Cyst Complicate ASD?
- Fred Shaffer
- Oct 13, 2025
- 23 min read

Dr. Ronald Swatzyna, Director and Chief Scientist of the Houston Neuroscience Brain Center, inspired our Clinician Detective series. In his Association for Applied Psychophysiology and Biofeedback (AAPB) Distinguished Scientist address, he reminded his audience that the DSM-5 advises that general medical conditions be systematically ruled out before assigning a psychiatric diagnosis to ensure diagnostic validity and appropriate treatment planning. He argued that in abrupt onset and refractory cases, EEG biomarkers should challenge neurofeedback providers and their medical colleagues to become detectives to identify its causes. This collaborative approach allows each professional to contribute to assessment while "staying in their lane."

Dr. Swatzyna generously mentors professionals in his investigative method, including raw EEG interpretation, to train the next generation of neurofeedback clinicians.
This post is based on Dr. Swatzyna's thought-provoking BCIA webinar, "Neurofeedback Mystery Theater #1. Two Cases with Atypical Findings and Neurofeedback Response." We encourage you to purchase this neurofeedback mentoring webinar from BCIA.

Clinical History and ASD Diagnosis
This case involves a 6-year-old male with a confirmed diagnosis of Autism Spectrum Disorder (ASD), characterized by persistent challenges in social communication and the presence of restricted, repetitive patterns of behavior, interests, or activities. The diagnosis of ASD was complicated by comorbid apraxia and hypotonia.
Apraxia in this context refers to a neurodevelopmental motor planning disorder known as Childhood Apraxia of Speech (CAS), which prevents the brain from sending correct signals to the muscles required for speech. This child showed significant difficulty initiating speech movements despite understanding language, a key feature of apraxia.
Hypotonia, or decreased muscle tone, resulted in loose, floppy muscle groups and poor postural control, making it difficult for the child to maintain sitting posture for long durations or engage in coordinated motor tasks like holding a crayon or climbing stairs. Both apraxia and hypotonia added layers of complexity to the ASD diagnosis and contributed to developmental delays in both fine and gross motor skills.
Importantly, this child had undergone multiple assessments and interventions from infancy, yet the severity and persistence of symptoms indicated an underlying biological mechanism not fully addressed by behavioral therapies alone. His family pursued genetic testing, which revealed a rare FOXP4 gene variant. FOXP4 (Forkhead Box Protein P4) encodes a transcription factor involved in neurogenesis, speech-language development, and cortical organization.
Variants in this gene are associated with language delay, cognitive impairments, and ASD-like presentations. Because the FOXP4 gene is active in early embryonic development, mutations may exert effects prenatally, suggesting that the root of the child's challenges lay in early neurodevelopment. The presence of this gene variant may explain the limited impact of standard interventions and highlights the importance of precision medicine in neurodevelopmental care. This initial diagnostic framing emphasized the necessity for a multidisciplinary and genomically informed approach.
Further complicating the picture, the child presented with significant chronic behavioral dysregulation that was unresponsive to medication and traditional behavioral interventions. The diagnostic process included structured developmental assessments, school-based evaluations, and neuropsychological testing. These evaluations confirmed deficits in expressive and receptive language, executive function, and sensory integration.
While his symptoms met full criteria for ASD, the level of functional impairment suggested additional etiological contributors. His history of nonresponse to stimulants and mood stabilizers further reinforced the need to explore non-psychiatric origins for his behavioral profile. Thus, the identification of the FOXP4 variant was more than a diagnostic detail; it marked a turning point in the case formulation, shifting the clinical emphasis toward genomics, mitochondrial assessment, and integrative medicine.
Developmental and Behavioral Observations
From early infancy, this child presented with behavioral cues that gradually signaled significant developmental concerns. He was described by his caregivers as unusually quiet and self-absorbed during his first year of life, lacking the social responsiveness typical in neurotypical infants. While he achieved gross motor milestones such as sitting and walking within expected timeframes, his social-emotional development lagged noticeably behind. He did not seek out social games like peek-a-boo, seldom responded to his name, and showed little interest in shared experiences. As he transitioned into toddlerhood, the absence of joint attention, imitation, and early play schemas became increasingly apparent.
Stimming behaviors were a predominant feature by age 2. These self-stimulatory behaviors occurred daily and increased in frequency during periods of excitement or distress. The most frequently observed stimming patterns included vigorous hand flapping, toe-walking, clenching of the fists with open-mouthed tension, and repetitive jumping. He also displayed oral-motor stemming (e.g., tongue protrusions and jaw tightening), which was interpreted as a manifestation of his sensory-seeking profile. These behaviors served regulatory functions, helping him manage both sensory input and emotional arousal. Behavioral specialists noted that these patterns were exacerbated in environments with excessive sensory stimulation, such as loud classrooms or large family gatherings.
The child's language development remained significantly delayed despite intensive early interventions. He was functionally nonverbal at 6 years of age, with occasional use of a speech-generating device to request items. He rarely initiated communication and often required prompts to engage even in basic interactive routines. Evaluators described a dissociation between his receptive and expressive language abilities; while he appeared to comprehend familiar commands and routines, he had considerable difficulty producing spontaneous language. He relied heavily on gestures, pulling caregivers' hands or guiding them physically to indicate needs. His inability to express himself verbally contributed to episodes of frustration, particularly during transitions or when denied preferred items.
Sensory sensitivities were also marked. He was hyperreactive to auditory stimuli, covering his ears in response to loud sounds like toilets flushing or applause. Visual overstimulation was evident as well, with the child frequently shielding his eyes when exposed to flickering lights or bright sunlight. Conversely, he displayed sensory-seeking behaviors such as a strong preference for deep pressure (e.g., tight hugs) and rhythmic motion (e.g., swinging or being rocked). These observations informed his occupational therapy treatment plan, which focused on sensory integration strategies to support regulation.
Overall, his behavioral profile was consistent with a diagnosis of ASD, but the presence of apraxia and hypotonia added complexity. His behaviors did not respond predictably to behavioral reinforcement strategies. For instance, token economies and contingency-based prompting yielded inconsistent results. His emotional dysregulation, marked by episodic aggression, tearfulness, and withdrawal, was exacerbated by fatigue or physiological discomfort, further suggesting a neurobiological component to his behavioral expression. These findings supported a shift away from purely behavioral frameworks toward a more integrated biopsychosocial model of care.
Educational and Therapeutic Interventions
The child was enrolled in a public elementary school and qualified for an Individualized Education Plan (IEP) designed to meet his diverse developmental and educational needs. His IEP included specialized academic instruction, speech-language therapy, occupational therapy, and behavioral support services. Modifications to the academic environment included shortened instructional segments, visual schedules, sensory accommodations, and the provision of a 1:1 classroom aide. Although he was mainstreamed for some activities, he spent the majority of his school day in a self-contained classroom designed for students with moderate to severe communication disorders. Teachers noted strengths in visual learning and nonverbal reasoning tasks but reported persistent deficits in sustained attention, symbolic play, and reciprocal social interaction.
To address these challenges, a comprehensive therapy regimen was implemented. He received two 30-minute sessions per week each of speech therapy and occupational therapy, focusing on augmentative communication and sensory integration respectively.
Speech therapy emphasized pragmatic communication skills and the use of a speech-generating device, while occupational therapy incorporated proprioceptive input, vestibular activities, and fine motor coordination tasks.
Behaviorally, the child was supported through a Positive Behavior Intervention Plan (PBIP), which utilized antecedent modifications, visual cues, and structured reinforcement schedules to reduce outbursts and increase engagement. Despite these supports, progress was slow, and teachers reported high variability in his ability to self-regulate and attend to tasks.
In an effort to address suspected neurological underpinnings of his limited progress, neurofeedback training was initiated. Neurofeedback, a form of operant conditioning, involves placing EEG sensors on the scalp to monitor brainwave activity and providing visual or auditory feedback to help individuals modulate their own cortical states. A total of 55 neurofeedback sessions were conducted over a 10-month period, targeting sensorimotor rhythm (SMR) enhancement and frontal midline theta reduction, protocols commonly used for ASD and attentional regulation. Treatment sessions occurred twice weekly and were conducted under medical supervision using standardized qEEG-informed protocols.
Parents reported initial improvements in sleep latency, eye contact, and tolerance for transitions during the first 3 months of treatment. These gains appeared to plateau but remained stable throughout the course of intervention. However, following the conclusion of neurofeedback, the child exhibited notable behavioral regression including increased irritability, loss of toileting gains, and frequent emotional meltdowns. These post-termination effects suggested that while neurofeedback did not produce overtly transformative effects, it may have provided a stabilizing influence on his neural regulation.
Additionally, the family pursued experimental stem cell therapy in the form of a single umbilical cord blood infusion. Cord blood is rich in hematopoietic stem cells and immunomodulatory cytokines and has shown preliminary promise in modulating inflammatory processes implicated in ASD. The procedure was performed under compassionate use guidelines in a clinical trial framework. Post-infusion, subtle behavioral improvements were noted by parents, including increased responsiveness to social cues and reduced frequency of stimming behaviors. However, these changes were not captured in formal assessments, and the causality of improvement remains uncertain.
The multidisciplinary treatment approach, though robust and personalized, did not yield proportional developmental progress. This outcome prompted deeper investigation into underlying neurobiological contributors, including genetic and metabolic etiologies. It also raised important questions about the sustainability and generalization of neurofeedback-related gains and highlighted the challenges in isolating treatment effects in cases involving simultaneous multimodal interventions.
Neurophysiological Findings
The child underwent two comprehensive EEG assessments spaced several months apart to evaluate cortical function and help guide treatment planning. Electroencephalography (EEG) is a noninvasive method of measuring the brain's electrical activity using scalp electrodes. It captures waveforms across various frequency bands—delta, theta, alpha, beta, and gamma—each reflecting different aspects of brain function.
The initial EEG showed a posterior dominant rhythm (PDR) centered at 7.8 Hz, which is below the expected norm for a 6-year-old. In typically developing children, the PDR should fall between 8.5 to 10.5 Hz by age 6, indicating cortical maturation. The alpha rhythm was poorly formed and unstable, suggestive of delayed neurodevelopment.
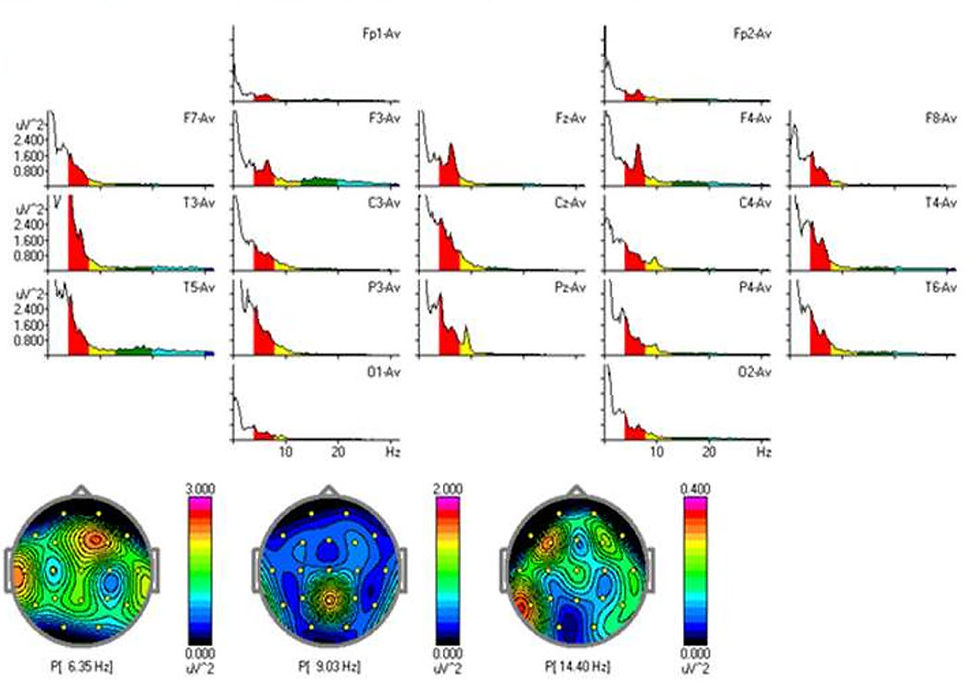
Additionally, the average EEG microvoltage was approximately 8.7 microvolts (µV), which is considered suboptimal. In this age group, a healthy brain typically generates 10–20 µV, depending on arousal state and cortical activity. Low-voltage EEGs are often associated with mitochondrial dysfunction or global cortical underactivation. Temporal slowing was observed bilaterally, particularly in the right posterior temporal region. This type of waveform abnormality may point to underlying white matter dysregulation or local cortical disorganization, such as that seen in cortical dysplasia. No clear epileptiform activity (e.g., spikes, sharp waves) was detected, though asynchronous theta bursts and semi-rhythmic slowing patterns raised clinical concern.
The follow-up EEG, conducted approximately 9 months later, demonstrated subtle but encouraging changes. The alpha peak had increased to 8.06 Hz, and microvoltage had risen to 11 µV. Despite these gains, background organization remained suboptimal. Temporal slowing persisted, although with reduced amplitude. Importantly, the EEG continued to exhibit poor interhemispheric synchrony—a feature often seen in children with ASD and related neurodevelopmental disorders. Synchrony refers to the coordinated timing of neural activity across brain regions, and its absence may indicate impaired functional connectivity.
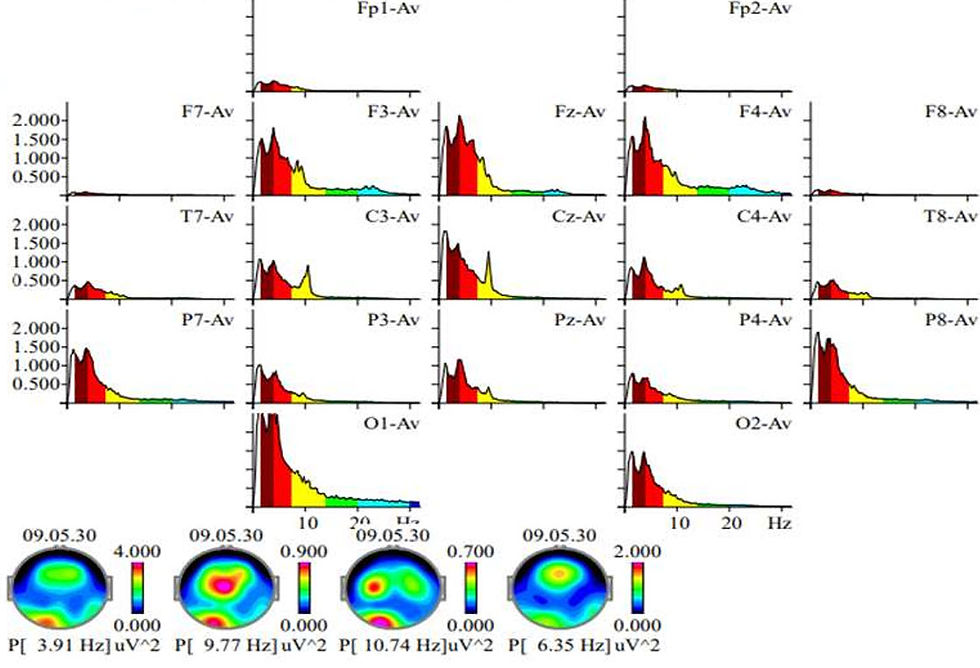
An automatic spike detector algorithm flagged several focal events over the central and frontal electrodes. However, upon manual review, these were not confirmed as epileptiform discharges. The reviewing neurologist described the EEG as "not normal but not diagnostic," recommending additional imaging studies to investigate structural contributions. A subsequent MRI revealed a benign pituitary-region cyst, which may or may not have relevance to the child’s symptoms. This case highlights the necessity of integrating EEG findings with clinical presentation, behavior, and neuroimaging to formulate a complete picture.
Neurofeedback protocols were adjusted following each EEG to reflect the most current neurophysiological profile. For example, initial sessions targeted increasing alpha coherence and reducing frontal theta activity. Later sessions emphasized stabilizing posterior dominant rhythm and supporting SMR activity over central sites. These adaptations were based on the theory that EEG normalization corresponds with improved cognitive and behavioral functioning, though empirical verification remains challenging due to the individualized nature of both EEG patterns and treatment responses.
FOXP4 and Metabolic Findings
The child’s clinical profile prompted a detailed investigation into possible genetic and metabolic contributors to his persistent developmental delays and non-responsiveness to traditional interventions. Genetic testing included chromosomal microarray analysis and whole exome sequencing. These tests are designed to identify deletions, duplications, and single nucleotide variants that may disrupt neurodevelopmental pathways. Results revealed a rare pathogenic variant in the FOXP4 gene. FOXP4 encodes a transcription factor involved in the regulation of neural progenitor differentiation, motor neuron specification, and development of the basal ganglia and frontal lobes. Mutations in FOXP4 are associated with impaired speech and language acquisition, hypotonia, and a phenotype consistent with syndromic autism.
The identification of the FOXP4 mutation provided an explanatory model for the child's profound apraxia, limited progress with expressive communication, and neurobehavioral rigidity. Importantly, FOXP4-related neurodevelopmental disorder does not follow a uniform trajectory, and phenotypic expression can vary widely, even among individuals with the same variant. This variability makes treatment outcomes difficult to predict. The presence of a gene mutation with known neurodevelopmental impact also helped reframe parental expectations and refocus clinical goals on functional support rather than neurotypical developmental benchmarks.
In parallel, metabolic screening revealed low serum carnitine levels. Carnitine is an essential cofactor in mitochondrial beta-oxidation, the process by which fatty acids are converted to cellular energy. Deficiency can impair mitochondrial function, particularly in energy-intensive organs such as the brain and heart. Carnitine deficiency in neurodevelopmental populations has been associated with fatigue, hypotonia, developmental regression, and low-voltage EEG patterns—all of which were present in this case. While primary carnitine deficiency is rare, secondary deficiency can result from dietary restriction, gastrointestinal dysbiosis, or chronic illness.
Supplementation with L-carnitine was initiated at a conservative dosage and titrated upward under metabolic supervision. The family noted modest improvements in energy, postural stability, and affective tone within the first 6–8 weeks. Although no dramatic cognitive gains were observed, the child's reduced irritability and improved tolerance for daily activities were clinically meaningful. These improvements coincided with stabilization in neurophysiological parameters, as evidenced by EEG, and a reduction in behavioral regression following cessation of neurofeedback.
Together, the genetic and metabolic data underscored the importance of considering systemic and cellular contributors to neurodevelopmental disorders. They demonstrated that structural brain anomalies, aberrant electrophysiology, and maladaptive behaviors may stem from gene-environment interactions that impair core processes such as energy metabolism, neural signaling, and circuit formation. By integrating these findings into the child’s case formulation, the clinical team was able to better tailor medical and educational interventions.
Medical and Structural Findings
Following persistent abnormalities in EEG and an inconsistent clinical trajectory, the child underwent magnetic resonance imaging (MRI) to identify potential structural contributors to his developmental presentation. MRI provides high-resolution images of brain anatomy, enabling detection of congenital anomalies, lesions, and abnormalities in white and gray matter. Imaging revealed a non-enhancing cystic lesion located near the sella turcica—the saddle-shaped depression at the base of the skull that houses the pituitary gland. This structure, known as a Rathke's cleft cyst, is typically benign and asymptomatic but can exert pressure on the pituitary gland or adjacent structures if enlarged.
The pituitary gland regulates critical hormonal pathways including growth hormone (GH), thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), and antidiuretic hormone (ADH). Disruptions in pituitary function may manifest as abnormal growth patterns, mood instability, sleep disturbances, and impaired stress regulation—symptoms consistent with those exhibited by the child. Despite the lesion being stable and not enhancing with contrast (which suggests a lack of malignancy), its proximity to the hypothalamic-pituitary axis raised concerns. A pediatric endocrinologist was consulted to evaluate pituitary function, and comprehensive hormonal panels were ordered to assess cortisol, free T4, TSH, IGF-1, and prolactin levels.
Although laboratory results were within reference ranges, the possibility of intermittent or subclinical dysfunction was not ruled out. Endocrine disturbances, particularly in the context of neurodevelopmental disorders, can present with subtle behavioral and cognitive symptoms long before overt signs of hormonal imbalance appear. The child’s above-average height and fluctuations in mood were noted as potentially relevant to pituitary activity. As a precaution, six-month follow-up imaging was recommended to monitor for cyst growth or changes in morphology. Additionally, serial endocrine evaluations were planned to identify any emerging dysfunction over time.
From a structural standpoint, the MRI did not reveal cortical malformations such as polymicrogyria, periventricular nodular heterotopia, or agenesis of the corpus callosum. However, mild ventricular asymmetry and a subtle thinning of the posterior corpus callosum were noted—findings that, while nonspecific, have been reported in association with ASD and global developmental delay. These features were considered incidental but contributory to the overall neurodevelopmental profile. Importantly, they reinforced the need for a holistic interpretation of anatomical, physiological, and behavioral data.
The medical findings—especially the presence of a sellar cyst and subclinical abnormalities—highlighted the importance of structural and systemic imaging in children with refractory developmental and behavioral concerns. While not pathognomonic, such findings may interact synergistically with genetic and metabolic vulnerabilities to exacerbate symptoms. The inclusion of structural imaging and endocrine evaluation represented a turning point in the child’s case management, underscoring the interdisciplinary nature of neurodevelopmental diagnostics.
Interpretative Challenges
This case presented numerous interpretative complexities, underscoring the limitations of relying on single modalities to understand neurodevelopmental dysfunction. One major challenge was disentangling the effects of concurrent interventions. The child received neurofeedback, speech therapy, occupational therapy, stem cell infusion, and L-carnitine supplementation—often simultaneously or in overlapping phases. Each modality targets different systems: neurofeedback aims to enhance cortical self-regulation, therapy addresses behavioral and sensory challenges, and medical interventions support cellular metabolism. The overlapping timelines made it difficult to attribute observed changes to any single intervention. For instance, improved social engagement and reduced emotional outbursts were noted after stem cell therapy and neurofeedback—but the lack of control conditions precluded causal inference.
Another interpretive difficulty involved variability in EEG findings. While the qEEG identified multiple abnormal features—low microvoltage, delayed alpha peak, and temporal slowing—none were definitively epileptiform. These ambiguous results, sometimes referred to informally as failing the “funny look test,” challenged standard classification. The patient exhibited abnormal synchrony, regional slowing, and background disorganization that did not align with clean diagnostic categories like epilepsy or encephalopathy.
Although not diagnostic in isolation, these patterns were clinically relevant and signaled atypical neurodevelopment. Manual review of the raw EEG was essential to rule out artifact and to verify the subtle anomalies flagged by automated software. This exemplified the value of direct waveform inspection over sole reliance on computed metrics.
Further complicating interpretation was the discovery of the FOXP4 mutation. The rarity of this variant meant limited published data existed to guide expectations or interpret symptoms. While the literature supported associations with speech delay, ASD features, and hypotonia, the phenotypic range remains poorly defined. Consequently, clinical teams were forced to make provisional judgments in the absence of genotype-specific guidelines. Similarly, the Rathke’s cleft cyst and borderline hormonal findings posed questions without clear answers. While not grossly abnormal, these findings contributed to a picture of biological vulnerability that likely interacts with genetic and metabolic factors.
The broader challenge in this case was synthesizing disparate lines of evidence—behavioral, electrophysiological, structural, genetic, and metabolic—into a coherent formulation. Each provided a piece of the puzzle but none offered a complete explanation. For example, low microvoltage EEG was consistent with mitochondrial dysfunction, but not sufficient to explain all behavioral features. Similarly, cortical thinning and ventricular asymmetry hinted at atypical brain development but lacked specificity. The challenge was not merely diagnostic but also prognostic: without clear biomarkers or trajectories, clinicians struggled to predict treatment responsiveness or long-term outcomes. These uncertainties complicated therapeutic planning and parent counseling.
Ultimately, the interpretive demands of this case reflected the complexity inherent in many pediatric neurodevelopmental profiles. The intersection of rare gene mutations, metabolic imbalances, subtle structural deviations, and ambiguous electrophysiological patterns required an integrative, interdisciplinary approach. It also highlighted the limits of current diagnostic frameworks, which often favor categorical over dimensional or multifactorial models. This case served as a compelling argument for personalized medicine, real-time hypothesis testing, and collaboration across specialties.
Clinical Recommendations
Given the multifaceted and treatment-resistant nature of this case, clinical recommendations emphasized a coordinated, interdisciplinary approach focused on both symptom management and underlying etiological contributors. Foremost was the referral for a full functional medicine evaluation.
Functional medicine examines root causes by integrating physiological, environmental, nutritional, and genomic data. This approach was warranted by the child’s overlapping signs of mitochondrial dysfunction, gastrointestinal dysbiosis, and neuroinflammatory risk—factors inadequately addressed by standard care. A functional medicine workup would likely include advanced panels assessing oxidative stress, detoxification pathways, gastrointestinal permeability, immune reactivity, and metabolic markers such as lactate, pyruvate, and organic acids.
Simultaneously, continued monitoring of pituitary function was recommended. While laboratory values were within normal ranges, the proximity of the Rathke’s cleft cyst to the hypothalamic-pituitary axis warranted serial endocrine evaluations. These would help detect subclinical or fluctuating hormone levels not captured in single assessments. Imaging follow-up every 6–12 months was also advised to rule out cyst growth, hemorrhage, or structural displacement. In tandem with endocrinological review, neurodevelopmental tracking should continue via updated psychological assessments, adaptive functioning measures, and real-world behavioral observations.
From a neurophysiological standpoint, further EEG monitoring was encouraged, ideally incorporating both eyes-closed and task-based protocols. Clinicians were advised to visually inspect raw EEG data in addition to reviewing quantitative maps. Particular attention should be paid to changes in interhemispheric synchrony, spectral power, and transient slowing events. A clinical neurologist and qEEG specialist should both be engaged for review, with a shared emphasis on distinguishing true cortical abnormalities from muscle artifact or environmental interference.
Regarding neurofeedback, a modified and extended protocol was proposed. Instead of terminating treatment after 55 sessions, a maintenance schedule could be introduced, with biweekly or monthly sessions focusing on SMR enhancement and stabilization of alpha rhythms. Such a strategy aims not for dramatic transformation but for ongoing neural support. If available, adjunctive technologies such as photobiomodulation—shown to enhance mitochondrial function and cerebral blood flow—could be explored as complementary interventions.
Educationally, the IEP should be revised to reflect emerging medical findings. This includes documentation of the FOXP4 variant and recommendations for embedded sensory regulation support, executive function coaching, and augmentative communication training.
School personnel should receive in-service education on the neurobiological aspects of ASD and the implications of metabolic and genetic comorbidities. Parents were encouraged to act as case coordinators, maintaining records, facilitating information exchange among providers, and advocating for reevaluations as new data emerge.
Psychological support for caregivers was also recommended given the emotional burden and uncertainty inherent in long-term, complex cases.
Finally, this case supported inclusion in a rare disorders registry or clinical trial database to facilitate research and longitudinal monitoring. Genomic data sharing, with appropriate consent, could contribute to improved understanding of FOXP4-related disorders and their treatment responsiveness. Future therapies—such as antisense oligonucleotides or CRISPR-based gene editing—might become viable, and early inclusion in registries may enable faster access when such interventions become available.
Timeline
Age 6
EEG Findings: Initial EEG showed low alpha peak at 7.8 Hz and average microvoltage of 8.7 µV. Semi-rhythmic temporal slowing and reduced interhemispheric synchrony were noted. No epileptiform discharges were observed.
Behavioral Findings: Significant speech delay, severe apraxia, hypotonia, self-stimulatory behaviors, and nonverbal status. Sensory sensitivities and emotional dysregulation were prominent.
Treatment: Initiated neurofeedback targeting SMR enhancement and alpha coherence. Early speech and occupational therapy continued. Behavioral plan established through IEP.
Age 6.5
EEG Findings: Follow-up EEG showed improvement in alpha peak to 8.06 Hz and microvoltage increased to 11 µV. Background organization remained poor with persistent temporal slowing.
Behavioral Findings: Subtle improvements in sleep, attention, and transitions. Nonverbal status persisted. Increased responsiveness to therapy and more engagement with caregivers.
Treatment: Received single stem cell infusion (cord blood). Continued neurofeedback and metabolic monitoring. Initiated carnitine supplementation.
Age 7
Structural Findings: MRI identified Rathke’s cleft cyst near pituitary gland. Endocrine labs normal but subclinical dysfunction not ruled out. Mild ventricular asymmetry and corpus callosum thinning noted.
Behavioral Findings: Episodes of emotional volatility and inconsistent academic engagement. Improvements in stamina and postural control observed.
Treatment: Pediatric endocrinologist involved. Regular follow-ups scheduled for neuroimaging and labs. Functional medicine evaluation initiated.
Age 7.5
EEG and Clinical Summary: Raw EEG reviewed due to re-emerging concerns. Subtle changes in coherence and theta activity monitored. Functional medicine identified carnitine deficiency and suspected mitochondrial dysfunction.
Behavioral Findings: Stabilization of emotional outbursts. Improved ability to tolerate new routines. Increased expressive attempts using AAC device.
Treatment: Continued multidisciplinary support. Neurofeedback sessions moved to monthly maintenance. Integration into specialized academic curriculum advanced.
Age 8
Summary: Overall behavioral profile showed modest, sustained improvement. Sleep, regulation, and attentional control improved. Functional medicine approach considered essential in guiding recovery trajectory.
Treatment: Maintenance neurofeedback continued. Expanded dietary interventions. Patient added to gene registry for FOXP4-related research.
Lessons Learned
This case illustrates the need for a truly integrative approach in pediatric neurodevelopmental assessment and intervention. One of the clearest takeaways is that surface-level behavioral observations, while essential, must be contextualized within deeper biological frameworks. In this child’s case, it was not sufficient to rely on traditional diagnostic categories such as ASD or ADHD. Only by combining the qEEG, genetic testing, metabolic panels, and neuroimaging could the interdisciplinary team appreciate the multifactorial etiology underpinning the presentation. Clinicians must resist the urge to isolate symptoms and instead develop diagnostic formulations that incorporate genomic, structural, and physiological layers.
A second key lesson is the limited utility of isolated treatment modalities. Neurofeedback, for example, provided temporary stabilization, but not lasting gains. Its value was better understood when positioned as one component of a long-term care strategy rather than a standalone intervention. Likewise, traditional school-based services, though helpful, required augmentation by therapies addressing cellular energy production, immune response, and gut-brain signaling. The child’s mild but significant response to L-carnitine supplementation reinforced the importance of exploring metabolic vulnerabilities.
Third, this case reinforced the diagnostic and prognostic value of longitudinal data. Repeated EEGs, follow-up MRIs, and structured behavioral observations revealed trends not visible at single time points. EEG findings became more interpretable when tracked over time, and subtle hormonal fluctuations were contextualized only through serial assessment. This suggests that developmental neurodiagnostics must evolve to incorporate time-based metrics, particularly in children with rare or overlapping conditions.
Finally, the case emphasized the power of collaboration—between disciplines, between data sources, and between clinicians and families. No single professional could have generated such a comprehensive understanding alone. Geneticists, neurologists, neurofeedback specialists, psychologists, educators, and caregivers each contributed indispensable perspectives. This reinforces the idea that successful outcomes in complex neurodevelopmental cases require not only precision tools but also interprofessional humility, curiosity, and shared problem-solving.
Key Takeaways
Neurodevelopmental complexity often reflects overlapping biological, genetic, and structural factors, not just behavioral symptoms.
Isolated interventions may yield limited results; integrated treatment plans grounded in systems medicine are essential.
Longitudinal monitoring enhances diagnostic clarity and informs treatment responsiveness across time.
EEG interpretation must combine quantitative and raw waveform analysis to avoid misinterpretation.
Genetic testing can clarify diagnostic ambiguity and reset treatment expectations for both families and providers.
Structural findings, even if incidental, may interact with metabolic or neurological vulnerabilities.
Functional medicine provides a framework to explore and treat root causes when standard care reaches its limits.
Interdisciplinary collaboration and case coordination are indispensable for high-complexity presentations.



Glossary
adaptive functioning: the set of practical, everyday skills required to function and meet environmental demands.
adrenocorticotropic hormone (ACTH): a hormone produced by the pituitary gland that stimulates cortisol release from the adrenal cortex.
alpha coherence: the degree to which alpha waves across different brain regions exhibit synchronized activity; higher coherence can reflect better communication between regions.
ambiguous EEG findings: non-specific patterns on EEG that are atypical but not clearly diagnostic, often requiring expert interpretation.
antisense oligonucleotides (ASOs): short, synthetic strands of nucleotides designed to bind to RNA and modify gene expression.
apraxia: a motor planning disorder where the brain has difficulty coordinating movements necessary for speech or other tasks, despite having the physical capacity to do so.
artifact (EEG): non-neural signal distortions in EEG recordings caused by external sources like muscle movement or electrode interference.
ASD (Autism Spectrum Disorder): a neurodevelopmental condition marked by difficulties in social communication and restricted, repetitive behaviors.
augmentative and alternative communication (AAC): strategies and tools that support individuals with severe speech or language impairments, including devices, picture boards, and gestures.
augmentative communication: any method of communication used to supplement or replace speech, including symbols, devices, or manual signs.
basal ganglia: a group of subcortical nuclei involved in motor control, behavior regulation, and procedural learning.
beta-oxidation: a metabolic process in mitochondria that breaks down fatty acids to produce ATP, the primary cellular energy molecule.
carnitine: a nutrient and metabolite that transports long-chain fatty acids into mitochondria for energy production.
chromosomal microarray: a genetic test that detects copy number variations (deletions or duplications) across the genome.
contingency-based prompting: behavioral technique that uses specific prompts to elicit desired behaviors, contingent on reinforcement.
corpus callosum: the large bundle of nerve fibers connecting the left and right cerebral hemispheres, crucial for interhemispheric communication.
cortical dysplasia: a congenital abnormality where the brain's cortical layers are malformed, often leading to developmental delay or seizure activity.
detoxification pathways: biological systems responsible for processing and eliminating toxins from the body, often assessed in functional medicine.
dimensional model: a conceptual framework that evaluates symptoms along a continuum rather than in discrete diagnostic categories.
EEG (Electroencephalogram): a method of recording the brain's electrical activity through sensors placed on the scalp.
electrophysiological pattern: characteristic waveforms or activity observed in EEG that may indicate brain function or dysfunction.
endocrine evaluation: testing and interpretation of hormonal function, often involving serial measurements to detect changes over time.
endocrinologist: a physician specializing in the diagnosis and treatment of hormone-related disorders.
exome sequencing: a genomic technique that sequences all protein-coding regions in the genome to identify mutations.
expressive language: the ability to produce language through speech, writing, or other communication.
expressive-receptive dissociation: a discrepancy where an individual's ability to understand language (receptive) exceeds their ability to express it (expressive).
FOXP4: a gene encoding a transcription factor critical for speech, motor, and cognitive development; mutations are associated with neurodevelopmental syndromes.
functional connectivity: the coordinated interaction between different areas of the brain, necessary for integrated processing.
functional medicine: a systems-based medical approach that seeks to identify and address root causes of disease by integrating physiological, genetic, and environmental data.
funny look test: informal term used by clinicians to describe EEGs that appear abnormal or inconsistent without meeting strict diagnostic criteria.
growth hormone (GH): a hormone produced by the pituitary gland that stimulates growth, cell reproduction, and regeneration.
hypothalamic-pituitary axis: the interactive neuroendocrine system that regulates stress response, metabolism, and reproductive function.
hypothesis testing (clinical): iterative process of generating and evaluating potential explanations for observed symptoms.
hypotonia: reduced muscle tone, which can result in motor delays and difficulties with posture.
Individualized Education Plan (IEP): a legally mandated document outlining special education services, goals, and accommodations for students with disabilities in U.S. public schools.
interdisciplinary approach: a treatment model involving collaboration across multiple professional domains to manage complex cases.
interhemispheric synchrony: the simultaneous timing of electrical activity between the left and right hemispheres of the brain.
joint attention: the shared focus of two individuals on an object or event, a foundational social-communicative skill often impaired in autism.
L-carnitine supplementation: medical treatment involving oral or intravenous administration of carnitine to correct deficiency.
magnetic resonance imaging (MRI): a noninvasive imaging technique that uses magnetic fields and radio waves to produce detailed images of internal structures, including the brain.
microvolt (µV): the unit of measurement for EEG amplitude, reflecting the strength of electrical signals generated by brain activity.
mitochondria: cellular organelles that produce energy through oxidative phosphorylation; dysfunction can impact neurological health.
mitochondrial dysfunction: impaired function of mitochondria, which produce cellular energy; associated with fatigue, developmental delays, and neurological symptoms.
neural progenitors: early-stage cells in the brain that give rise to neurons and glial cells during development.
occupational therapy (OT): therapeutic approach aimed at helping individuals perform everyday activities, with emphasis on sensory, motor, and cognitive skills.
oral-motor stemming: repetitive movements of the mouth and jaw used to self-regulate sensory input.
personalized medicine: an approach to treatment that tailors interventions based on individual genetic, physiological, and environmental factors.
phenotypic variability: differences in observable characteristics or traits among individuals with the same genetic condition.
photobiomodulation: a therapeutic technique that uses red or near-infrared light to stimulate cellular function and promote healing.
pituitary gland: a small endocrine gland at the base of the brain that controls the activity of other hormone-secreting glands.
Positive Behavior Intervention Plan (PBIP): a structured plan using proactive and reinforcement-based strategies to address challenging behaviors in educational settings.
posterior dominant rhythm (PDR): the primary alpha activity seen in the occipital region of the brain during relaxed wakefulness; a key indicator of cortical maturity.
prolactin: a pituitary hormone involved in lactation and other metabolic and immune functions.
proprioceptive input: sensory feedback from muscles and joints that informs the brain about body position, often used in therapeutic activities to improve regulation.
qEEG (Quantitative EEG): a computerized analysis of the EEG used to detect patterns that may be associated with specific neuropsychological conditions.
Rathke's cleft cyst: a benign, fluid-filled cyst that develops from remnants of Rathke’s pouch near the pituitary gland.
receptive language: the ability to understand language input, such as spoken or written words.
self-contained classroom: an educational setting where students with similar needs receive individualized instruction in a separate space from general education classrooms.
self-injurious behavior: harmful behaviors directed toward oneself, sometimes seen in individuals with ASD under conditions of stress or frustration.
semi-rhythmic slowing: EEG pattern characterized by irregular but repetitive slow-frequency activity, often seen in encephalopathic or immature brains.
sensorimotor rhythm (SMR): a type of brainwave (typically 12–15 Hz) associated with a calm, focused state; enhancing SMR is a common neurofeedback target for attention and motor regulation.
sensory integration: the neurological process that organizes sensation from the body and environment for effective use; often targeted in OT.
sella turcica: a saddle-shaped bony structure at the base of the skull that contains the pituitary gland.
speech-generating device: a form of augmentative and alternative communication (AAC) that produces spoken output to support nonverbal individuals.
stimming: self-stimulatory behavior often seen in autism, used to regulate sensory input or emotional states.
structural deviation: anatomical variations or irregularities in brain morphology that may or may not be symptomatic.
temporal slowing: reduced EEG frequency activity in the temporal lobes; may suggest focal cortical dysfunction.
transcription factor: a protein that regulates gene expression by binding to specific DNA sequences, thereby influencing cell function and identity.
vestibular activities: movements that stimulate the inner ear balance system, used in therapy to support body awareness and sensory processing.
voltage (EEG): the strength or amplitude of electrical activity measured in the brain, typically expressed in microvolts.
References
Alfano, C., Lepore, M., Amoroso, A., D'Angelo, D., Quaglietta, L., Coppola, G., Viggiano, D., & Di Salle, F. (2020). FOXP family transcription factors in neural development and disorders. International Journal of Molecular Sciences, 21(2), 605. https://doi.org/10.3390/ijms21020605
Courchesne, E., Campbell, K., & Solso, S. (2011). Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Research, 1380, 138–145. https://doi.org/10.1016/j.brainres.2010.09.101
Kumar, M., & Hasan, Q. (2021). Neurofeedback therapy: A comprehensive review on the applications and future prospects. Frontiers in Neuroscience, 15, 620164. https://doi.org/10.3389/fnins.2021.620164
Rossignol, D. A., & Frye, R. E. (2012). Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Molecular Psychiatry, 17(3), 290–314. https://doi.org/10.1038/mp.2010.136
Santos, M., Tekin, M., & De la Hoz, A. B. (2018). Clinical and molecular features of FOXP4-related disorder: A review. Clinical Genetics, 94(6), 526–532. https://doi.org/10.1111/cge.13401
Shoffner, J., Hyams, L., Langley, G. N., Cossette, S., Mylotte, K. M., Dale, J., & Boles, R. G. (2009). Fever plus mitochondrial disease could be risk factors for autistic regression. Journal of Child Neurology, 24(4), 482–486. https://doi.org/10.1177/0883073808328682
Thompson, M., & Thompson, L. (2015). The neurofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Van Der Meer, D., Hartman, C. A., & Greven, C. U. (2020). The importance of gene-environment interactions in neurodevelopmental disorders. Current Opinion in Behavioral Sciences, 36, 1–7. https://doi.org/10.1016/j.cobeha.2020.06.002
About the Author

Fred Shaffer earned his PhD in Psychology from Oklahoma State University. He earned BCIA certifications in Biofeedback and HRV Biofeedback. Fred is an Allen Fellow and Professor of Psychology at Truman State University, where has has taught for 50 years. He is a Biological Psychologist who consults and lectures in heart rate variability biofeedback, Physiological Psychology, and Psychopharmacology. Fred helped to edit Evidence-Based Practice in Biofeedback and Neurofeedback (3rd and 4th eds.) and helped to maintain BCIA's certification programs.
Support Our Friends








Comments